Fexofenadine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- FEXOFENADINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- PHARMACODYNAMICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- FEXOFENADINE HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- FEXOFENADINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
FEXOFENADINE HYDROCHLORIDE DESCRIPTION
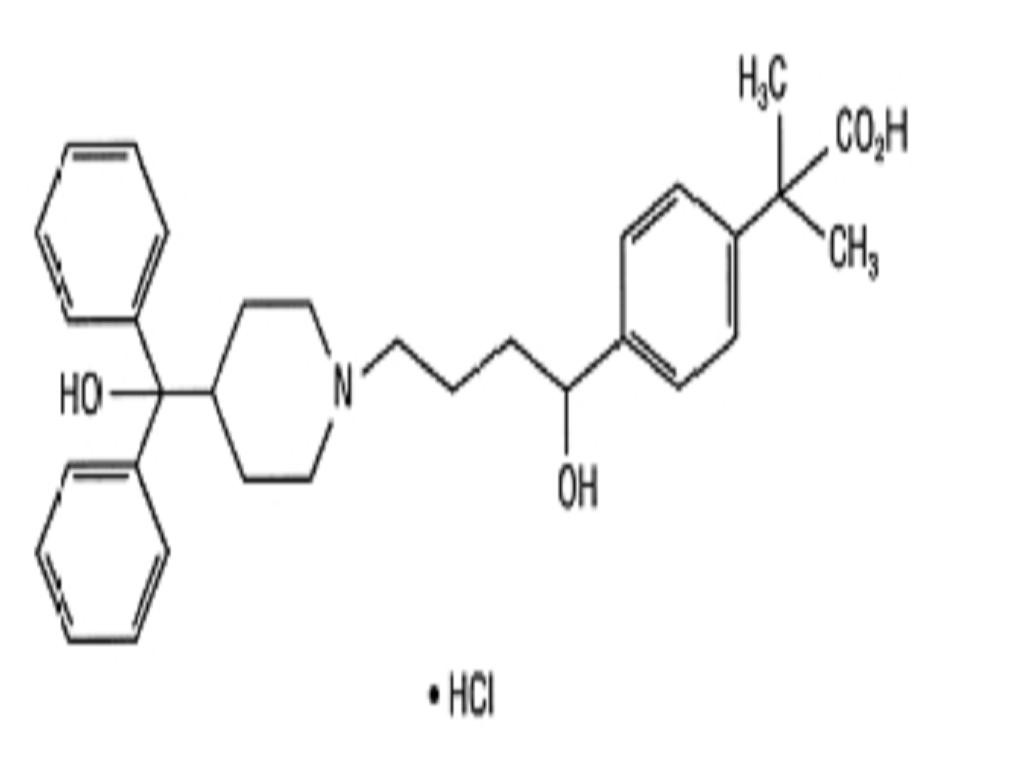
CLINICAL PHARMACOLOGY
Mechanism of Action:PHARMACOKINETICS
Absorption:Distribution:
Elimination:
Metabolism:
Special Populations:
Seasonal allergic rhinitis (SAR) and chronic idiopathic urticaria (CIU) patients:
Geriatric Subjects:
Pediatric Patients:
Renal Impairment:
Hepatic Impairment:
Effect of Gender:
PHARMACODYNAMICS
Wheal and Flare:Effects on QTC:
CLINICAL STUDIES
Seasonal Allergic Rhinitis:Adults:
Pediatrics:
Chronic Idiopathic Urticaria:
INDICATIONS & USAGE
Seasonal Allergic Rhinitis:Chronic Idiopathic Urticaria:
FEXOFENADINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS
Drug Interaction with Erythromycin and Ketoconazole:Drug Interactions with Antacids:
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects:Category C:
Nonteratogenic Effects:
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
FEXOFENADINE HYDROCHLORIDE ADVERSE REACTIONS
Seasonal Allergic Rhinitis:
Adults:
Pediatric:
Chronic Idiopathic Urticaria:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Seasonal Allergic Rhinitis:Adults and Children 12 Years and Older:
Children 6 to 11 Years:
Chronic Idiopathic Urticaria:
Adults and Children 12 Years and Older:
Children 6 to 11 Years:
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
-
● Fexofenadine hydrochloride tablets, are prescribed for the relief of symptoms of seasonal allergic rhinitis or for the relief of symptoms of chronic idiopathic urticaria (hives). Instruct patients to take fexofenadine hydrochloride only as prescribed. Do not exceed the recommended dose. If any untoward effects occur while taking fexofenadine hydrochloride, discontinue use and consult a doctor.
-
● Patients who are hypersensitive to any of the ingredients should not use these products.
-
● Patients who are pregnant or nursing should use these products only if the potential benefit justifies the potential risk to the fetus or nursing infant.
-
● Advise patients and parents/caregivers of pediatric patients to store the medication in a tightly closed container in a cool, dry place, away from small children.
-
● Advise patients and parents/caregivers not to take fexofenadine hydrochloride tablets with fruit juices.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Fexofenadine HydrochlorideFexofenadine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!