Fem pH
Fem pH™ Therapeutic Vaginal Jelly
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Fem pH Indications and Usage
- Contraindications
- Warnings
- Precautions
- Side Effects
- Dosage and Administration
- How Supplied
- Therapeutic Vaginal Jelly
- PRINCIPAL DISPLAY PANEL - 50g Tube Box
FULL PRESCRIBING INFORMATION
Rx Only
Description
Fem pH Vaginal Jelly is a bland, non-irritating water dispersible, buffered acid jelly for intravaginal use. Fem pH is classified as a Vaginal Therapeutic Jelly. Fem pH contains 0.9% glacial acetic acid (C2H402) and 0.025% oxyquinoline sulfate (C18H16N206S) compounded with glycerin, lactic acid, poly ethylene glycol 4500 and purified water. Fem pH is formulated to pH 3.8-4.3 and is adjusted using 1 N potassium hydroxide.
Clinical Pharmacology
Fem pH acts to restore and maintain normal vaginal acidity through its buffered action.
Fem pH Indications and Usage
Fem pH is indicated as adjunctive therapy in those cases where restoration and maintenance of vaginal acidity is desirable.
Contraindications
None known.
Warnings
No serious adverse reactions or potential safety hazard have been reported with the use of Fem pH.
Precautions
General
No special care is required for the safe and effective use of Fem pH.
Drug Interactions
No incidence of drug interactions has been reported with concomitant use of Fem pH and any other medication.
Laboratory Tests
The monitoring of vaginal acidity (pH) may be helpful in following the patient's response. (The normal vaginal pH has been shown to be in the range of 4.0 to 5.0)
Carcinogenesis
No long-term studies in animals have been performed to evaluate carcinogenic potential.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with Fem pH. It is not known whether Fem pH can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Fem pH should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Fem pH is administered to a nursing woman.
Side Effects
Occasional cases of local stinging and burning have been reported.
Dosage and Administration
The usual dose is one applicator full, administered intra-vaginally, morning and evening. Duration of treatment may be determined by the patient's response to therapy. Each tube has a tamper evident seal at the opening of the tube. Replace cap after each use. To fill applicator screw applicator clockwise onto the tube. Squeeze tube forcing Fem pH jelly into barrel until it is full. Then unscrew applicator counter-clockwise to remove from tube. Lie on your back with knees drawn up. Hold filled applicator by the barrel and gently insert it into the vagina as far as it will comfortably go. Press plunger to empty the contents. Keep the plunger depressed and remove the applicator from vagina. After each use pull applicator apart and wash with warm soapy water, rinse well, dry and reassemble.
How Supplied
50g Tube (NDC 00813-0799-55) with Fem pH applicator.
Keep this and all medication out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Store at controlled room temperature 59°-86°F (15°-30°C)
Mfg. by:
Pernix Manufacturing
Houston, TX 77099
Mfg. for:
Pharmics, Inc.
Salt Lake City, UT 84119
(801) 966-4138
www.pharmics.com
Revised 05/13
Therapeutic Vaginal Jelly
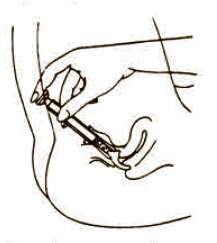
Directions for using Fem pH applicator.
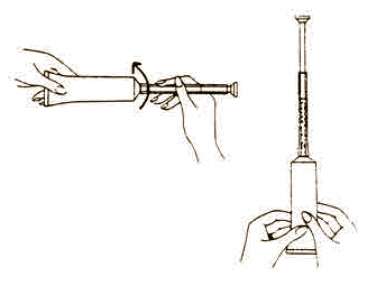
Each tube has a tamper evident seal at the opening of the tube. Replace cap after each use.
|
To fill applicator screw applicator clockwise onto the tube. Squeeze tube forcing Fem pH jelly into barrel until it is full. Then unscrew applicator counter-clockwise to remove from tube. Lie on your back with knees drawn up. Hold filled applicator by the barrel and gently insert it into the vagina as far as it will comfortably go. Press plunger to empty the contents. Keep the plunger depressed and remove the applicator from vagina. |
After each use pull applicator apart and wash with warm soapy water, rinse well, dry and reassemble.

PRINCIPAL DISPLAY PANEL - 50g Tube Box
NDC 00813-0799-55
Rx Only
Fem pH™
Therapeutic Vaginal Jelly
Net Weight 50g
(1.66 oz.)
pharmicsINC

Fem pHAcetic Acid and Oxyquinoline sulfate JELLY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||