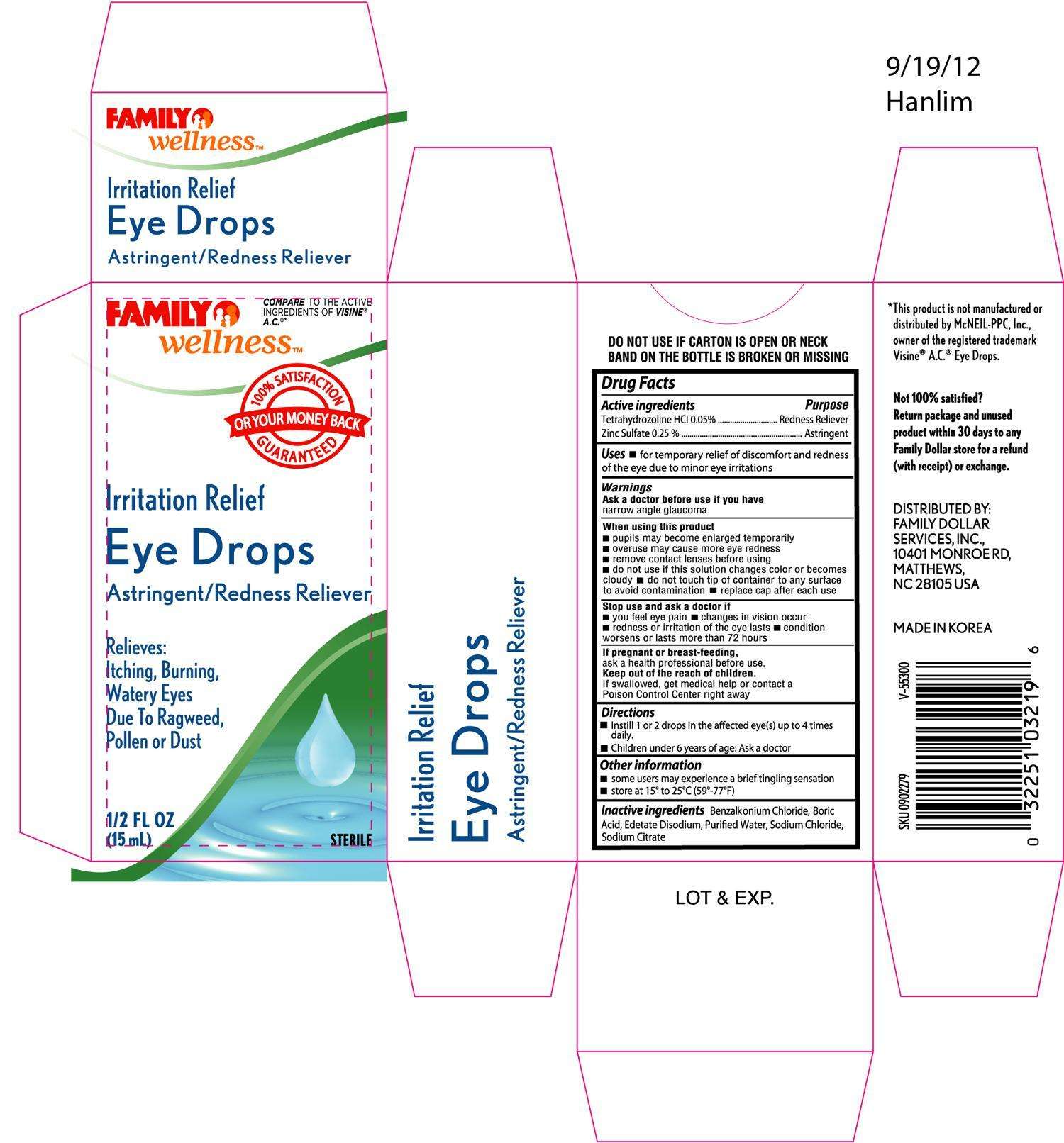
FAMILY WELLNESS IRRITATION RELIEF AC EYE DROPS
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Tetrahydrozoline HCL 0.05% ...........................................................Redness Reliever
Zinc Sulfate 0.25% .........................................................................Astringent
Purpose
Uses
- for temporary relief of discomfort and redness of the eye due to minor eye irritations
Warnings
Ask a doctor before use if you have narrow angle glaucoma
When using this product
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- do not use if this solution changes color or become cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness or irritation of the eye lasts
- condition worsens or lasts more than 72 hours
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
Directions
- Instill 1 or 2 drops in the affected eye(s) up to 4 times daily
- Children under 6 years of age: Ask a doctor
Inactive ingredients: Benzalkonium Chloride, Boric Acid, Edetate Disodium, Purified Water, Sodium Chloride, Sodium Citrate
DISTRIBUTED BY:
FAMILY DOLLAR SERVICES, INC.
10401 MONROE RD
MATTHEWS, NC 28105 USA
MADE IN KOREA
Other information
- some users may experience a brief tingling sensation
- store at 15o to 25oC (59o-77oF)
FAMILY WELLNESS IRRITATION RELIEF AC EYE DROPSTETRAHYDROZOLINE HYDROCHLORIDE AND ZINC SULFATE SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||