Erythrocin Lactobionate
ERYTHROCIN™ Lactobionate - IVErythromycin Lactobionate for Injection, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ERYTHROCIN LACTOBIONATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- ERYTHROCIN LACTOBIONATE INDICATIONS AND USAGE
- ERYTHROCIN LACTOBIONATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ERYTHROCIN LACTOBIONATE ADVERSE REACTIONS
- OVERDOSAGE
- ERYTHROCIN LACTOBIONATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
FULL PRESCRIBING INFORMATION
Single-dose
ADD-VantageTM Vials
For Intravenous Use Only
Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
ERYTHROCIN LACTOBIONATE DESCRIPTION
ErythrocinTM Lactobionate-IV (erythromycin lactobionate for injection, USP) is a lyophilized powder for intravenous infusion only. It is prepared as a solution and lyophilized in its final container. The Erythrocin Lactobionate-IV ADD-VantageTM vial is designed for use only with the ADD-Vantage Flexible Diluent Container. After appropriate dilution, the Erythrocin Lactobionate-IV ADD-Vantage Delivery System contains erythromycin lactobionate equivalent to either 500 mg of erythromycin activity in 100 mL.
The solutions contain no bacteriostat, antimicrobial agent (except erythromycin) or buffer and are intended for use as a single-dose injection only with the ADD-Vantage Flexible Diluent Container.
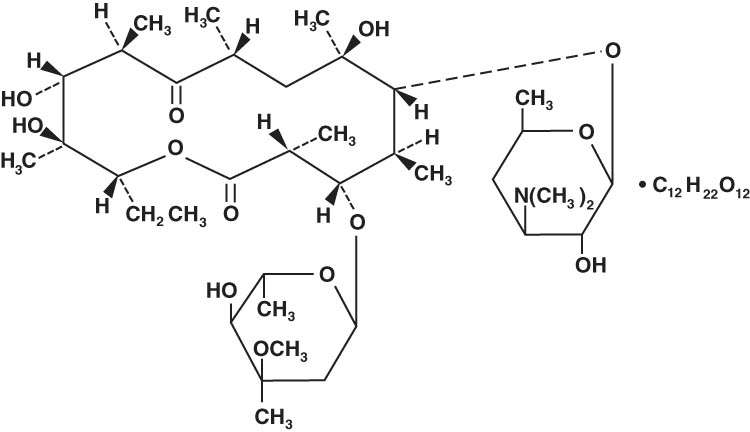
Erythromycin is produced by a strain of Streptomyces erythraeus and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids. Erythromycin lactobionate has the following structure:
CLINICAL PHARMACOLOGY
Erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid but the passage of the drug across the blood-brain barrier increases in meningitis. Erythromycin crosses the placental barrier and is excreted in breast milk. Erythromycin is not removed by peritoneal dialysis or hemodialysis.
In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. From 12 to 15 percent of intravenously administered erythromycin is excreted in active form in the urine.
Intravenous infusion of 500 mg of erythromycin lactobionate at a constant rate over 1 hour in fasting adults produced a mean serum erythromycin level of approximately 7 mcg/mL at 20 minutes, 10 mcg/mL at 1 hour, 2.6 mcg/mL at 2.5 hours, and 1 mcg/mL at 6 hours.
Microbiology
Erythromycin is a macrolide antibiotic with activity against Gram-positive and Gram-negative bacteria.
Mechanism of Action
Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis.
Interactions with Other Antibiotics
Antagonism has been demonstrated in vitro between erythromycin and clindamycin, lincomycin and chloramphenicol.
Many strains of Haemophilus influenzae are resistant to erythromycin, but are susceptible to erythromycin and sulfonamides used concomitantly.
Development of Resistance
Resistance to erythromycin in S. aureus may emerge during therapy.
Erythromycin has been shown to be active against most strains of the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE ):
Gram-positive bacteria Aerobic
Corynebacterium diphtheriae
Corynebacterium minutissimum
Staphylococcus aureus
(methicillin-susceptible strains only)
Streptococcus pneumoniae
Streptococcus pyogenes
Gram-negative bacteria
Legionella pneumophila
Neisseria gonorrhoeae
Other Microorganisms
Mycoplasma pneumonia
At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for erythromycin. However, the efficacy of erythromycin in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled trials.
Gram-negative bacteria
Moraxella catarrhalis
Susceptibility Testing
When available, the clinical microbiology laboratory should provide cumulative results of the in vitro susceptibility test results for antimicrobial drugs used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth or agar)1 or equivalent with standardized inoculum concentrations and standardized concentrations of erythromycin powder. The MIC values should be interpreted according to the criteria provided in Table 1.
Diffusion technique
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 15 mcg of erythromycin to test the susceptibility of microorganisms to erythromycin. The disk diffusion interpretive criteria are provided in Table 1.
|
Pathogen |
Susceptibility Interpretive Criteria |
|||||
|
Minimum Inhibitory |
Disk Diffusion |
|||||
|
S |
I |
R |
S |
I |
R |
|
|
Staphylococcus spp . |
≤0.5 |
1 to 4 |
≥8 |
≥23 |
14 to 22 |
≤13 |
|
Enterococcus spp. |
≤0.5 |
1 to 4 |
≥8 |
≥23 |
14 to 22 |
≤13 |
|
Streptococcus pneumoniae a,b |
≤0.25 |
0.5 |
≥1 |
≥21 |
16 to 20 |
≤15 |
|
Streptococcus
spp. |
≤0.25 |
0.5 |
≥1 |
≥21 |
16 to 20 |
≤15 |
|
Streptococcus
spp. |
≤0.25 |
0.5 |
≥1 |
≥21 |
16 to 20 |
≤15 |
|
a The MIC interpretive criteria for Streptococcus pneumoniae , Streptococcus spp. (β-hemolytic group), and Streptococcus spp. (Viridans group) are applicable only to tests performed by broth microdilution using cation-adjusted Mueller-Hinton broth supplemented with 2 to 5% lysed horse blood inoculated with a direct colony suspension and incubated in ambient air at 35 ± 2°C for 20 to 24 hours. b The zone diameter interpretive criteria for Streptococcus pneumoniae , Streptococcus spp. (β‑hemolytic group), and Streptococcus spp. (Viridans group) are applicable only to tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood inoculated with a direct colony suspension and incubated in 5% CO2 at 35 ± 2°C for 20 to 24 hours. |
||||||
A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of Intermediate indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone, which prevents small, uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound in the blood reaches the concentrations usually achievable and other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of quality control microorganisms to control the technical aspects of the test procedures3. Standard Erythromycin powder should provide the following range of values noted in Table 2.
|
QC Strain |
Acceptable Quality Control Ranges |
|
|
Minimum Inhibitory |
Disk Diffusion |
|
|
Enterococcus faecalis |
1 to 4 |
NAa |
|
Staphylococcus aureus |
0.25 to 1 |
NA |
|
Staphylococcus aureus |
NA |
22 to 30 |
|
Streptococcus pneumoniae |
0.03 to 0.12c |
25 to 30d |
|
a not applicable b This organism may be used for validation of susceptibility test results when testing Streptococcus spp. other than S. pneumoniae . c This quality control range for S. pneumoniae is applicable only to tests performed by broth microdilution using cation-adjusted Mueller-Hinton broth supplemented with 2 to 5% lysed horse blood inoculated with a direct colony suspension and incubated in ambient air at 35 ± 2°C for 20 to 24 hours. d This quality control zone diameter range is applicable only to tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood inoculated with a direct colony suspension and incubated in 5% CO2 at 35 ± 2°C for 20 to 24 hours. |
||
ERYTHROCIN LACTOBIONATE INDICATIONS AND USAGE
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is indicated in the treatment of infections caused by susceptible strains of the designated organisms in the diseases listed below when oral administration is not possible or when the severity of the infection requires immediate high serum levels of erythromycin. Intravenous therapy should be replaced by oral administration at the appropriate time.
Upper respiratory tract infections of mild to moderate degree caused by Streptococcus pyogenes (Group A beta-hemolytic streptococci); Streptococcus pneumoniae (Diplococcus pneumoniae) ; Haemophilus influenzae (when used concomitantly with adequate doses of sulfonamides, since many strains of H. influenzae are not susceptible to the erythromycin concentrations ordinarily achieved). (See appropriate sulfonamide labeling for prescribing information.)
Lower respiratory tract infections of mild to moderate severity caused by Streptococcus pyogenes (Group A beta-hemolytic streptococci); Streptococcus pneumoniae (Diplococcus pneumoniae) .
Respiratory tract infections due to Mycoplasma pneumoniae .
Skin and skin structure infections of mild to moderate severity caused by Streptococcus pyogenes and Staphylococcus aureus (resistant staphylococci may emerge during treatment).
Diphtheria: As an adjunct to antitoxin infections due to Corynebacterium diphtheriae to prevent establishment of carriers and to eradicate the organism in carriers.
Erythrasma: In the treatment of infections due to Corynebacterium minutissimum .
Acute pelvic inflammatory disease caused by Neisseria gonorrhoeae : Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) followed by erythromycin stearate or erythromycin base orally, as an alternative drug in treatment of acute pelvic inflammatory disease caused by N. gonorrhoeae in female patients with a history of sensitivity to penicillin.
Before treatment of gonorrhea, patients who are suspected of also having syphilis should have a microscopic examination for T. pallidum (by immunofluorescence or darkfield) before receiving erythromycin and monthly serologic tests for a minimum of 4 months thereafter.
Legionnaires’ Disease caused by Legionella pneumophila . Although no controlled clinical efficacy studies have been conducted, in vitro and limited preliminary clinical data suggest that erythromycin may be effective in treating Legionnaires’ Disease.
Prevention of Initial Attacks of Rheumatic Fever
Penicillin is considered by the American Heart Association to be the drug of choice in the prevention of initial attacks of rheumatic fever (treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract e.g., tonsillitis, or pharyngitis).4 Erythromycin is indicated for the treatment of penicillin-allergic patients. The therapeutic dose should be administered for ten days.
Prevention of Recurrent Attacks of Rheumatic Fever
Penicillin or sulfonamides are considered by the American Heart Association to be the drugs of choice in the prevention of recurrent attacks of rheumatic fever. In patients who are allergic to penicillin and sulfonamides, oral erythromycin is recommended by the American Heart Association in the long-term prophylaxis of streptococcal pharyngitis (for the prevention of recurrent attacks of rheumatic fever).4
Prevention of Bacterial Endocarditis
Although no controlled clinical efficacy trials have been conducted, oral erythromycin has been recommended by the American Heart Association for prevention of bacterial endocarditis in penicillin-allergic patients with prosthetic cardiac valves, most congenital cardiac malformations, surgically constructed systemic pulmonary shunts, rheumatic or other acquired valvular dysfunction, idiopathic hypertrophic subaortic stenosis (IHSS), previous history of bacterial endocarditis and mitral valve prolapse with insufficiency when they undergo dental procedures and surgical procedures of the upper respiratory tract.5
To reduce the development of drug-resistant bacteria and maintain the effectiveness of erythromycin and other antibacterial drugs, erythromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
ERYTHROCIN LACTOBIONATE CONTRAINDICATIONS
Erythromycin is contraindicated in patients with known hypersensitivity to this antibiotic. Erythromycin is contraindicated in patients taking terfenadine or astemizole. (See PRECAUTIONS – Drug Interactions .)
WARNINGS
There have been reports of hepatic dysfunction, with or without jaundice occurring in patients receiving oral erythromycin products.
Clostridium difficile associated diarrhea (CDAD) has been reported with the use of nearly all antibacterial agents, including erythromycin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile , and surgical evaluation should be instituted as clinically indicated.
Life-threatening episodes of ventricular tachycardia associated with prolonged QT interval (torsades de pointes) have been reported in some patients after intravenous administration of erythromycin lactobionate.
Susceptibility to the development of torsades de pointes arrhythmias, a rare but serious cardiac condition, is related to electrolyte imbalance, hepatic dysfunction, myocardial ischemia, left ventricular dysfunction, idiopathic Q-T prolongation, and concurrent antiarrhythmic therapy.6 Elderly patients exhibit a greater frequency of decreased hepatic function, cardiac function, and of concomitant disease and other drug therapy, and therefore should be monitored carefully during ErythrocinTM therapy.
PRECAUTIONS
General
Since erythromycin is principally excreted by the liver, caution should be exercised when erythromycin is administered to patients with impaired hepatic function. (See CLINICAL PHARMACOLOGY and WARNINGS .)
There have been reports that erythromycin may aggravate the weakness of patients with myasthenia gravis.
Prolonged or repeated use of erythromycin may result in an overgrowth of non-susceptible bacteria or fungi. If superinfection occurs, erythromycin should be discontinued and appropriate therapy instituted.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy.
Prescribing erythromycin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Laboratory Tests
Erythromycin interferes with the fluorometric determination of urinary catecholamines.
Drug Interactions
Erythromycin use in patients who are receiving high doses of theophylline may be associated with an increase of serum theophylline levels and potential theophylline toxicity. In case of theophylline toxicity and/or elevated serum theophylline levels, the dose of theophylline should be reduced while the patient is receiving concomitant erythromycin therapy.
There have been published reports suggesting that when oral erythromycin is given concurrently with theophylline there is a significant decrease in erythromycin serum concentrations. This decrease could result in subtherapeutic concentrations of erythromycin.
Erythromycin administration in patients receiving carbamazepine has been reported to cause increased serum levels of carbamazepine with subsequent development of signs of carbamazepine toxicity.
Concomitant administration of erythromycin and digoxin has been reported to result in elevated serum digoxin levels.
There have been reports of increased anticoagulant effects when erythromycin and oral anticoagulants were used concomitantly.
Erythromycin has been reported to significantly alter the metabolism of the nonsedating antihistamines, terfenadine and astemizole, when taken concomitantly. Rare cases of serious cardiovascular adverse events, including electrocardiographic QT/QTc interval prolongation, cardiac arrest, torsades de pointes, and other ventricular arrhythmias, have been observed. (See CONTRAINDICATIONS .) In addition, deaths have been reported rarely with concomitant administration of terfenadine and erythromycin.
The use of erythromycin in patients concurrently taking drugs metabolized by the cytochrome P450 system may be associated with elevations in serum levels of these other drugs. There have been reports of interactions of erythromycin with carbamazepine, cyclosporine, hexobarbital, phenytoin, alfentanil, disopyramide, lovastatin, bromocriptine, valproate, terfenadine, and astemizole. Serum concentrations of drugs metabolized by the cytochrome P450 system should be monitored closely in patients concurrently receiving erythromycin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal data with erythromycin lactobionate for use in determination of possible carcinogenic effects are not available. However, long-term oral studies in rats with erythromycin ethylsuccinate and erythromycin base did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25% of diet.
Pregnancy
Pregnancy Category B
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25% of diet) prior to and during mating, during gestation, and through weaning of two successive litters. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Erythromycin has been reported to cross the placental barrier in humans, but fetal plasma levels are generally low.
Labor and Delivery
The effect of erythromycin on labor and delivery is unknown.
Nursing Mothers
Erythromycin is excreted in breast milk. Caution should be exercised when erythromycin is administered to a nursing woman.
Pediatric Use
(See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION ).
Geriatric Use
Elderly patients, particularly those with reduced renal or hepatic function, may be at increased risk for developing erythromycin-induced hearing loss, when ErythrocinTM doses of 4 grams/day or higher are given. (See ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION .)
Elderly patients may be more susceptible to the development of torsades de pointes arrhythmias than younger patients. (See ADVERSE REACTIONS .)
Elderly patients may experience increased effects of oral anticoagulant therapy while undergoing treatment with ErythrocinTM. (See PRECAUTIONS, Drug Interactions .)
Erythromycin Lactobionate does not contain sodium.
Information for Patients
Patients should be counseled that antibacterial drugs including erythromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When erythromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by erythromycin or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
ERYTHROCIN LACTOBIONATE ADVERSE REACTIONS
Erythromycin has been associated with QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes. (See WARNINGS .)
Side effects following the use of intravenous erythromycin are rare. Occasional venous irritation has been encountered, but if the infusion is given slowly, in dilute solution, preferably by continuous intravenous infusion or intermittent infusion in no less than 20 to 60 minutes, pain and vessel trauma are minimized.
Allergic reactions ranging from urticaria to anaphylaxis have occurred. Skin reactions ranging from mild eruptions to erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported rarely.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency and in patients receiving high doses of erythromycin.
Elderly patients, particularly those with reduced renal or hepatic function, may also be at increased risk for developing this effect when ErythrocinTM doses of 4 grams/day or higher are given. (See DOSAGE AND ADMINISTRATION .)
OVERDOSAGE
In the case of overdosage, erythromycin infusion should be discontinued and all other appropriate measures should be instituted.
Erythromycin is not removed by peritoneal dialysis or hemodialysis.
ERYTHROCIN LACTOBIONATE DOSAGE AND ADMINISTRATION
For the treatment of severe infections in adults and pediatric patients, the recommended intravenous dose of erythromycin lactobionate is 15 to 20 mg/kg/day. Higher doses, up to 4 g/day, may be given for severe infections.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function. Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) in the ADD-Vantage system must be administered by continuous or intermittent intravenous infusion only. Due to the irritative properties of erythromycin, IV push is an unacceptable route of administration.
Continuous infusion of erythromycin lactobionate is preferable due to the slower infusion rate and lower concentration of erythromycin; however, intermittent infusion at six hour intervals is also effective. Intravenous erythromycin should be replaced by oral erythromycin as soon as possible.
The drug should be administered as a single dose from the ADD-Vantage flexible diluent container. Discard any unused portion.
For slow continuous infusion: The final diluted solution of erythromycin lactobionate is prepared to give a concentration of 1 g per liter (1 mg/mL).
For intermittent infusion: administer one-fourth the total daily dose of erythromycin lactobionate by intravenous infusion in 20 to 60 minutes at intervals not greater than every six hours. The final diluted solution of erythromycin lactobionate is prepared to give a concentration of 1 to 5 mg/mL. No less than 100 mL of IV diluent should be used. Infusion should be sufficiently slow to minimize pain along the vein.
For treatment of acute pelvic inflammatory disease caused by N. Gonorrhoeae , in female patients hypersensitive to penicillins, administer 500 mg erythromycin lactobionate every six hours for three days, followed by oral administration of 250 mg erythromycin stearate or base every six hours for seven days.
For treatment of Legionnaires’ Disease: Although optimal doses have not been established, doses utilized in reported clinical data were 1 to 4 grams daily in divided doses.
Administration of doses of ≥4 g/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function.
In the treatment of Group A beta-hemolytic streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for ten days. The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides.4
In prophylaxis against bacterial endocarditis (see INDICATIONS AND USAGE ) the oral regimen for penicillin allergic patients is erythromycin 1 gram, 1 hour before the procedure followed by 500 mg six hours later.5
Preparation of Solution:
The Erythrocin Lactobionate-IV ADD-Vantage vial may be used with either 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP in the ADD-Vantage flexible diluent container. The 500 mg ADD-Vantage vials must be used as single doses with the 100 mL ADD-Vantage flexible diluent containers. The resulting solution will contain erythromycin activity equal to approximately 5 mg/mL.
Do not administer unless solution is clear and container is undamaged. Discard any unused portion.
INSTRUCTIONS FOR USE
To Use Vial in ADD-Vantage Flexible Diluent Container
To Open:
Peel overwrap at corner and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible Diluent Container:
(Use Aseptic Technique)
- Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
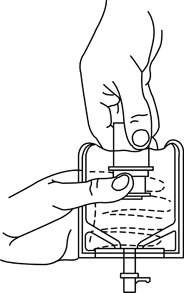
a. To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (See FIGURE 1.), then pull straight up to remove the cap. (See FIGURE 2.) NOTE: Do not access vial with syringe.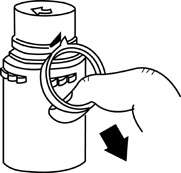

Fig. 1
Fig. 2
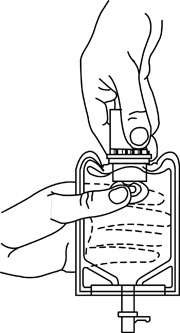
b. To remove the vial port cover, grasp the tab on the pull ring, pull up to break the three tie strings, then pull back to remove the cover. (See FIGURE 3.) - Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately 1/2 turn (180°) after the first audible click. (See FIGURE 4.) The clicking sound does not assure a seal; the vial must be turned as far as it will go.
NOTE: Once vial is seated, do not attempt to remove. (See FIGURE 4.)

Fig. 3
Fig. 4
- Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
- Label appropriately.
To Reconstitute the Drug:
- Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
- With the other hand, push the drug vial down into the container telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container. (See FIGURE 5.)
- Pull the inner cap from the drug vial. (See FIGURE 6.) Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
- Mix container contents thoroughly and use within the specified time.
|
|
|
|
Fig. 5 |
Fig. 6 |
Preparation for Administration:
(Use Aseptic Technique)
- Confirm the activation and admixture of vial contents.
- Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
- Close flow control clamp of administration set.
- Remove cover from outlet port at bottom of container.
- Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated.
NOTE: See full directions on administration set carton. - Lift the free end of the hanger loop on the bottom of the vial, breaking the two tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
- Squeeze and release drip chamber to establish proper fluid level in chamber.
- Open flow control clamp and clear air from set. Close clamp.
- Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
- Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible containers in series connections.
Stability:
In 0.9% Sodium Chloride Injection, USP
The final diluted solution of erythromycin lactobionate should be completely administered
within 8 hours
in order to assure proper potency.
In 5% Dextrose Injection, USP
The final diluted solution of erythromycin lactobionate should be completely administered
within 2 hours
in order to assure proper potency.
No drug or chemical agent should be added to an Erythrocin Lactobionate-IV fluid admixture unless its effect on the chemical and physical stability of the solution has first been determined.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
Erythrocin Lactobionate-IV (erythromycin lactobionate for injection, USP) is supplied as a sterile, lyophilized powder as follows:
|
NDC No. |
Container |
Concentration |
Quantity |
|
0409-6476-44 |
Single-dose ADD-Vantage Vial |
500 mg |
Package of 10 |
Store at 20 to 25ºC (68 to 77ºF). [See USP Controlled Room Temperature.]
REFERENCES
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-9th Edition. Clinical and Laboratory Standards Institute document M07-A9. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087-1898 USA, 2012.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-11th Edition . Clinical and Laboratory Standards Institute document M02-A11. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087-1898 USA, 2012.
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement. CLSI document M100-S22. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087-1898 USA, 2012.
- Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease of the Young: Prevention of Rheumatic Fever, Circulation 70(6):1118A-1122A, December 1984.
- Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease of the Young: Prevention of Bacterial Endocarditis, Circulation 70(6):1123A-1127A, December 1984.
- Gilter, B., et al, Torsades de Pointes Induced by Erythromycin, Chest, Volume 105: 368-72, February 1994.
Revised: 01/2013
EN-3199
Hospira, Inc., Lake Forest, IL 60045 USA
CA-3243 (front)

CA-3243 (back)

Erythrocin LactobionateERYTHROMYCIN LACTOBIONATE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Erythrocin LactobionateERYTHROMYCIN LACTOBIONATE INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||