Ecolab
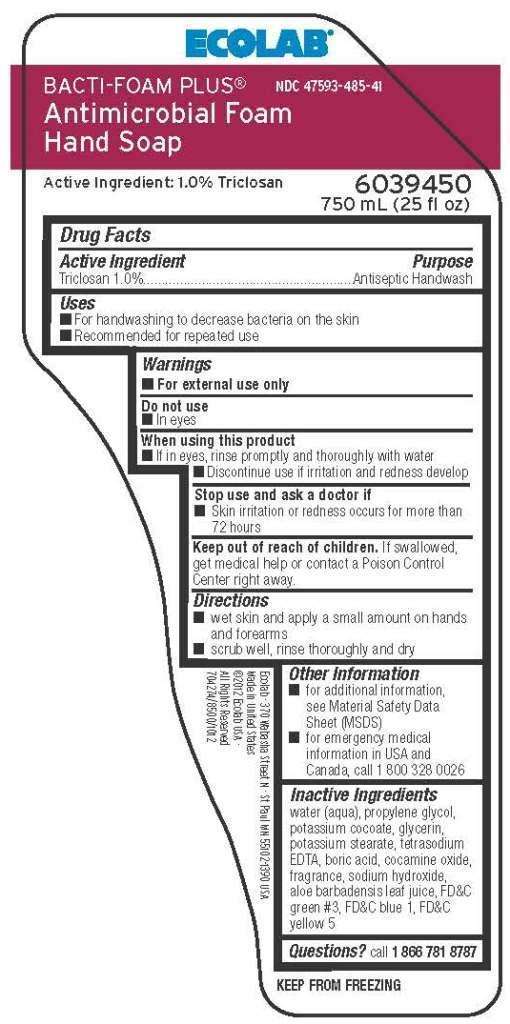
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Ecolab Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Principal Display Panel and Representative Label
FULL PRESCRIBING INFORMATION
Active Ingredient
Triclosan 1.0%
Purpose
Antiseptic Handwash
Ecolab Uses
- For handwashing to decrease bacteria on the skin
- Recommended for repeated use
Warnings
- For external use only
Do not use
- In eyes
When using this product
- If in eyes, rinse promptly and thoroughly with water
- Discontinue use if irritation and redness develop
Stop use and ask a doctor if
- Skin irritation or redness occurs for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact Poison Control Center right away.
Directions
- wet skin and apply a small amount on hands and forearms
- scrub well, rinse thoroughly and dry
Other Information
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA and Canada, call 1 800 328 0026
Inactive Ingredients
water (aqua), propylene glycol, potassium cocoate, glycerin, potassium stearate, tetrasodium EDTA, boric acid, cocamine oxide, fragrance, sodium hydroxide, aloe barbadensis leaf juice, FDC green #3, FDC blue 1, FDC yellow5
Questions? call 1 866 781 8787
Principal Display Panel and Representative Label
ECOLAB
BACTI-FOAM PLUS®
NDC 47593-485-41
Antimicrobial Foam Hand Soap
Active Ingredient: 1.0% Triclosan
6039450
750 mL (25 fl oz)
Ecolab - 370 Wabasha Street N - St Paul MN 55102-1390 USA
Made in United States
©2012 Eclab USA
All Rights Reserved
704274/8500/1012
KEEP FROM FREEZING
EcolabTriclosan SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||