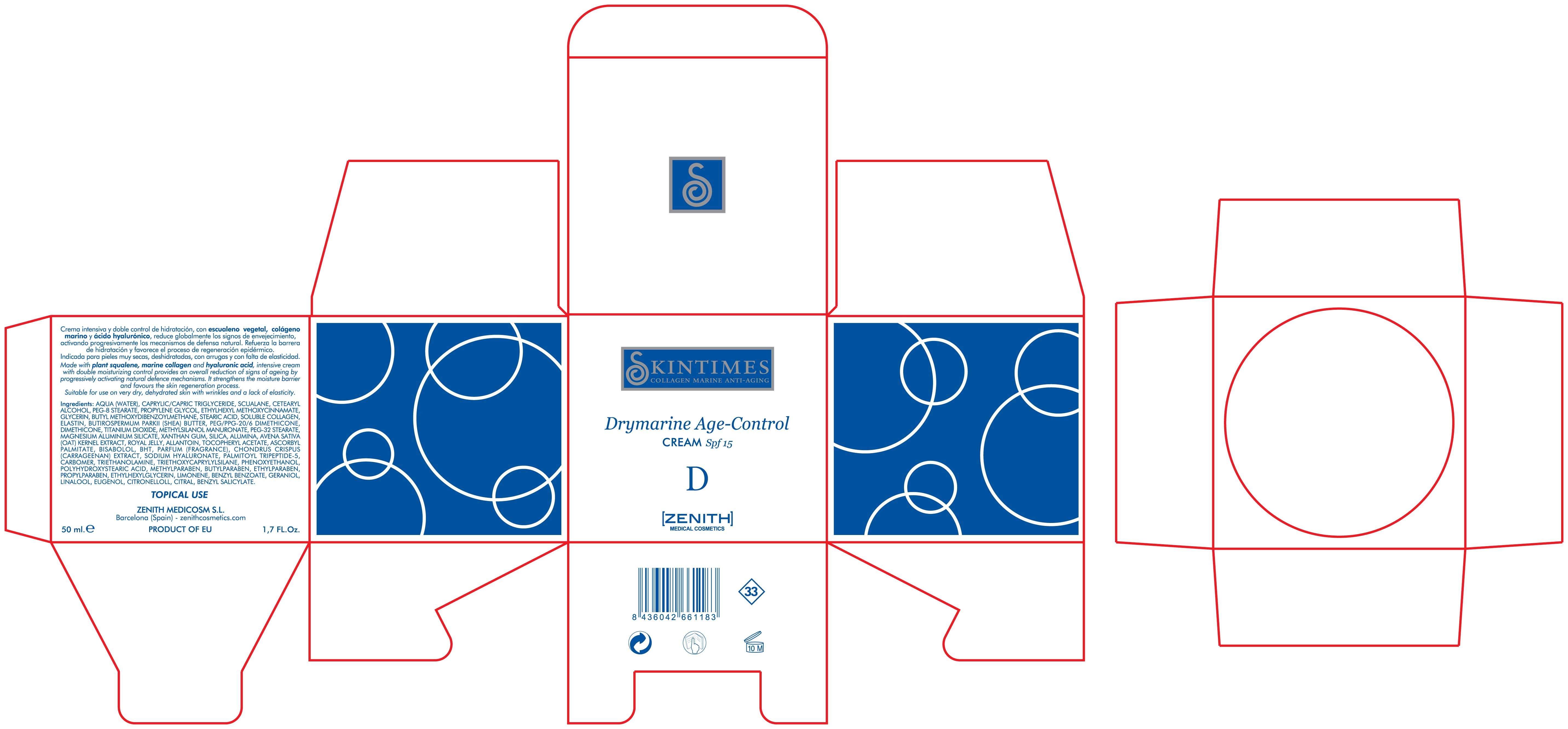
Drymarine
Zenith Medicosm SL
Zenith Medicosm SL
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredient
Octinoxate 7.5%
Titanium Dioxide 25%
Avobenzone 3%
Description
Made with plant squalene, marine collagen and hyaluronic acid, intensive cream with double moisturizing control provides an overall reduction of signs of ageing by progressively activating natural defence mechanisms. It strenghtens the moisture barrier and favours the skin regeneration process. Suitable for use on very dry, dehydrated skin with wrinkles and lack of elasticity.
50ml. 1.7 fl. Oz
Warning
TOPICAL USE
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!