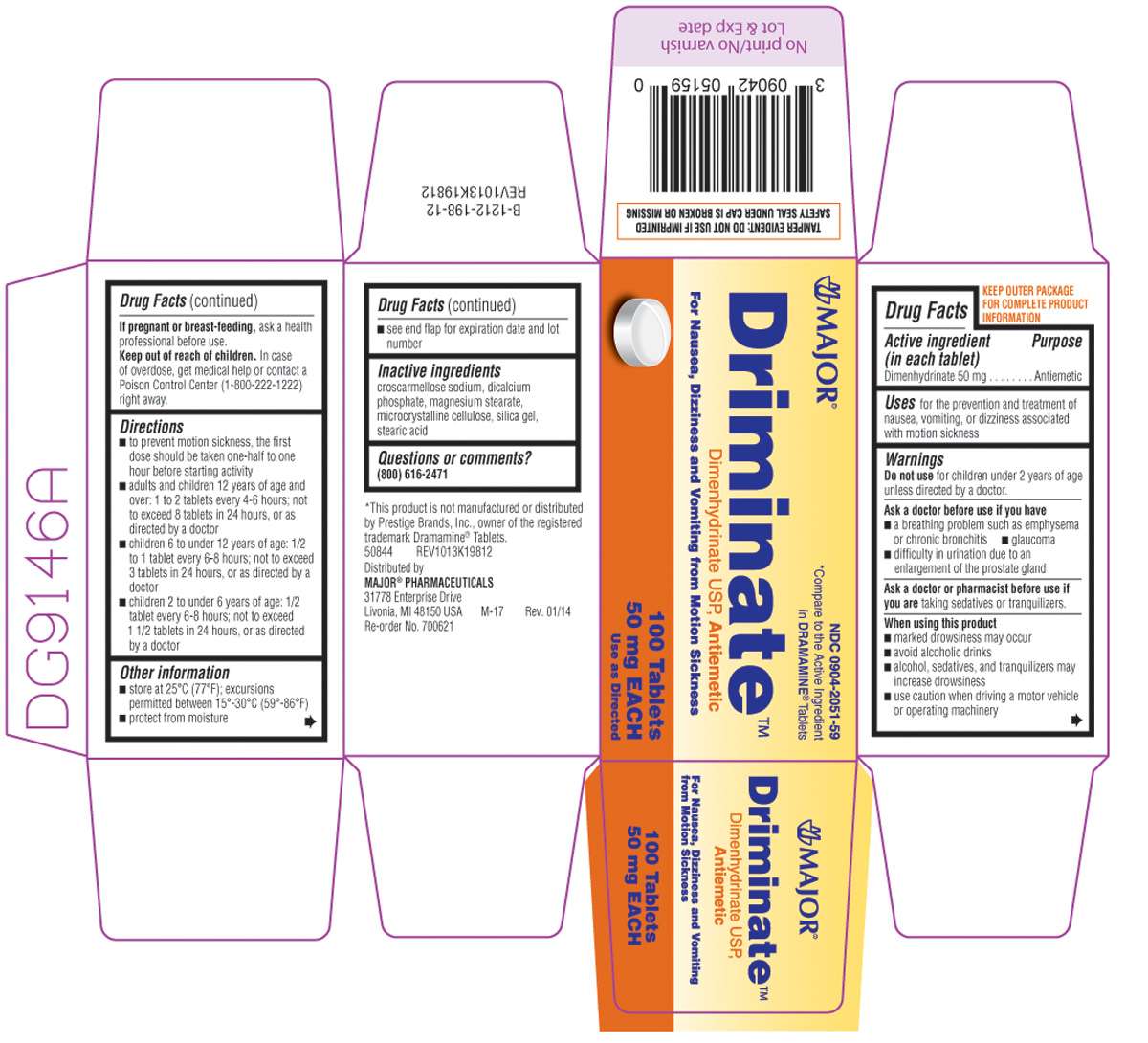
Driminate
Major 44-198
FULL PRESCRIBING INFORMATION
Dimenhydrinate 50 mg
Antiemetic
for the prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness
for children under 2 years of age unless directed by a doctor.
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
taking sedatives or tranquilizers.
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
- adults and children 12 years of age and over: 1 to 2 tablets every 4-6 hours; not to exceed 8 tablets in 24 hours, or as directed by a doctor
- children 6 to under 12 years of age: 1/2 to 1 tablet every 6-8 hours; not to exceed 3 tablets in 24 hours, or as directed by a doctor
- children 2 to under 6 years of age: 1/2 tablet every 6-8 hours; not to exceed 1 1/2 tablets in 24 hours, or as directed by a doctor
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from moisture
- see end flap for expiration date and lot number
croscarmellose sodium, dicalcium phosphate, magnesium stearate, microcrystalline cellulose, silica gel, stearic acid
(800) 616-2471
MAJOR®
NDC 0904-2051-59
*Compare to the Active Ingredient in DRAMAMINE® Tablets
Driminate™
Dimenhydrinate USP, Antiemetic
For Nausea, Dizziness and Vomiting from Motion Sickness
100 Tablets
50 mg EACH
Use as Directed
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine® Tablets.
50844 REV1013K19812
Distributed by MAJOR® PHARMACEUTICALS
31778 Enterprise Drive
Livonia, MI 48150 USA M-17
Re-order No. 700621
DriminateDimenhydrinate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||