Doxycycline Hyclate
FULL PRESCRIBING INFORMATION: CONTENTS*
- DOXYCYCLINE HYCLATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DOXYCYCLINE HYCLATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- DOXYCYCLINE HYCLATE ADVERSE REACTIONS
- DOXYCYCLINE HYCLATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DOXYCYCLINE HYCLATE DESCRIPTION
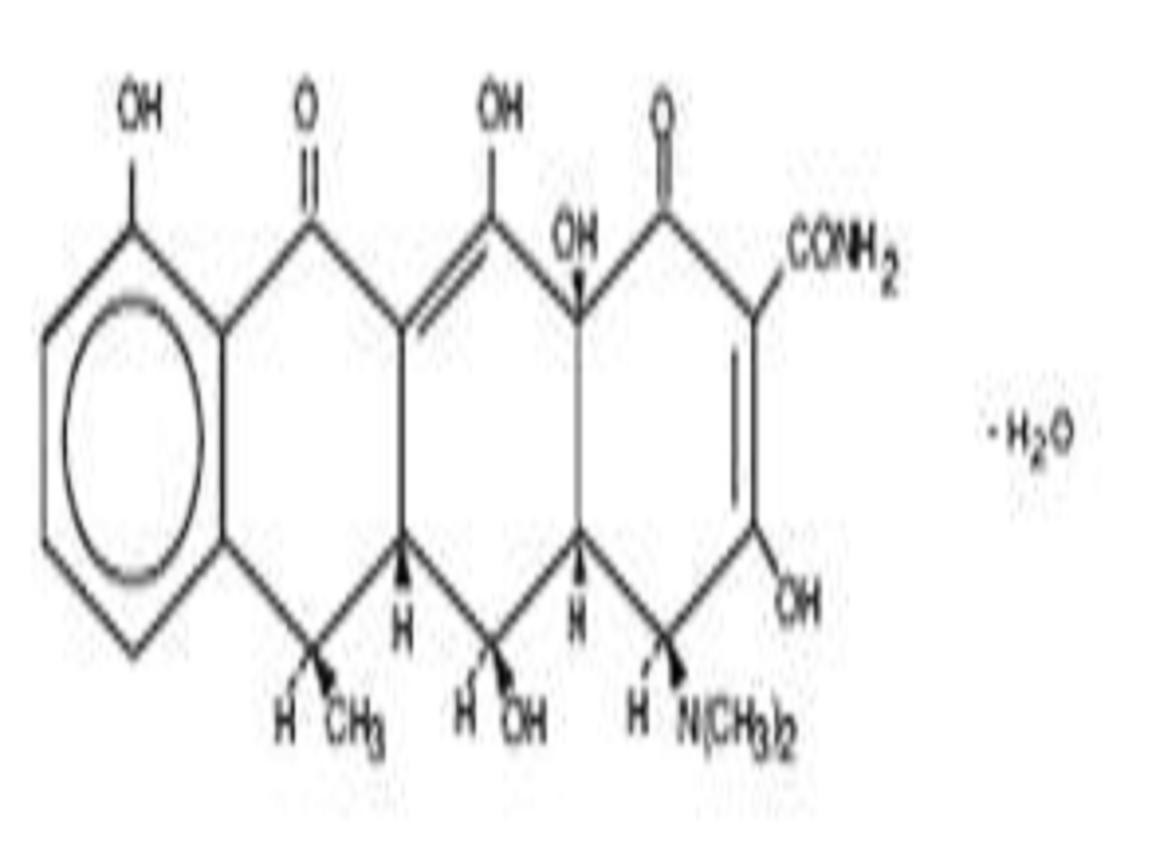
CLINICAL PHARMACOLOGY
Microbiology
Gram-Negative Bacteria
Gram-Positive Bacteria
Other Microorganisms
Susceptibility tests: Diffusion techniques:
Dilution techniques:
INDICATIONS & USAGE
Treatment:
-
● Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by Rickettsiae.
-
● Respiratory tract infections caused by Mycoplasma pneumoniae.
-
● Lymphogranuloma venereum caused by Chlamydia trachomatis.
-
● Psittacosis (omithosis) caused by Chlamydia trachomatis.
-
● Trachoma caused by Chlamydia trachomatis, although the infectious agent is not always eliminated as judged by immunofluorescence.
-
● Inclusion conjunctivitis caused by Chlamydia trachomatis.
-
● Uncomplicated urethral, endocervical or rectal infections in adults caused by Chlamydia trachomatis.
-
● Nongonococcal urethritis caused by Ureaplasma urealyticum.
-
● Relapsing fever due to Borrelia recurrentis.
-
● Chancroid caused by Haemophilius ducreyl.
-
● Plague due to Tersinia pestis (formerly Pasturella pestis).
-
● Tularemia due to Francisella tularensis (formerly Pasturella tularensis).
-
● Cholera caused by Vibrio cholerae (formerly Vibrio comma).
-
● Campylobacter fetus infections caused by Campylobacter fetus (formerly Vibrio fetus).
-
● Brucellosis due to Brucella species (in conjunction with streptomycin).
-
● Bartonellosis due to Bartonella bacilliformis.
-
● Granuloma inguinale caused by Calymmatobacterium granulomatis.
-
● Escherichia coli.
-
● Enterobacter aerogenes (formerly Aerobacter aerogenes).
-
● Shigella species.
-
● Acinetobacter species (formerly Mima species and Herellea species).
-
● Respiratory tract infections caused by Haemophilius influenzae.
-
● Respiratory tract and urinary tract infections caused by Klebsiella species.
-
● Upper respiratory infections caused by Streptococcus pneumoniae (formerly Diplococcus pneumoniae).
-
● Uncomplicated gonorrhea caused by Neisseria gonorrhoeae.
-
● Syphillis caused by Treponema pallidum.
-
● Yaws caused by Treponema pertenue.
-
● Listeriosis due to Listeria monocytogenes.
-
● Vincent's infection caused by Fusobacterium fusiforme.
-
● Actinomycosis caused by Actinomyces israelli.
-
● Infections caused by Clostridium species.
Prophylaxis:
DOSAGE AND ADMINISTRATIONInformation for PatientsPRECAUTIONS
DOXYCYCLINE HYCLATE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
WARNINGS
ADVERSE REACTIONS
Drug Interactions
Drug Interactions
WARNINGS
ADVERSE REACTIONS
Drug Interactions
Drug Interactions
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Nonteratogenic Effects: (See Warnings).
LABOR & DELIVERY
NURSING MOTHERS
WARNINGSPediatric Use
WARNINGSDOSAGE AND ADMINISTRATION
DOXYCYCLINE HYCLATE ADVERSE REACTIONS
DOSAGE AND ADMINISTRATION
WARNINGS
WARNINGS
PRECAUTIONSGeneral
DOXYCYCLINE HYCLATE ADVERSE REACTIONS
DOSAGE AND ADMINISTRATION
WARNINGS
WARNINGS
PRECAUTIONSGeneral
OVERDOSAGE
DOSAGE & ADMINISTRATION
ADVERSE REACTIONS
HOW SUPPLIED
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Doxycycline HyclateDoxycycline Hyclate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!