Doxycycline
General Injectables & Vaccines, Inc
Doxycycline 100 mg Injection, USP Single Dose Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- Doxycycline Indications and Usage
- Contraindications
- Warnings
- Precautions
- Side Effects
- Dosage and Administration
- How Supplied
- Sample Outer Label
FULL PRESCRIBING INFORMATION
Description
For IV Infusion Only
Rx ONLY
To reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline and other antibacterial drugs, doxycycline should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Doxycycline for Injection USP is a broad-spectrum antibiotic synthetically derived from oxytetracycline. It is a light yellow crystalline powder, and is available as doxycycline hydrochloride hemiethanolate hemihydrate. Chemically, doxycycline is 4- (DimethyIamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride, compound with ethyl alcohol (2:1), monohydrate. Doxycycline has a high degree of lipoid solubiIity and a low affinity for calcium binding. It is highly stable in normal human serum. Each vial contains a sterile mixture of doxycycline hyclate equivalent to 100 mg doxycycline and 480 mg ascorbic acid. The structural formula of Doxycycline Hyclate is as follows:
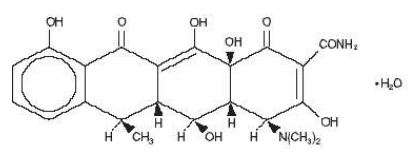
(C22H24N2O8•HCl)2•C2H5O•H2O M.W.=1025.87
Clinical Pharmacology
Doxycycline is primarily bacteriostatic and thought to exert its antimicrobial effect by the inhibition of protein synthesis. Doxycycline is active against a wide range of gram-positive and gram-negative organisms. The drugs in the tetracycline class have closely similar antimicrobial spectra, and cross resistance among them is common. Microorganisms may be considered susceptible to doxycycline (likely to respond to doxycycline therapy) if the minimum inhibitory concentration (M.I.C.) is not more than 4 mcg/mL. Microorganisms may be considered intermediate (harboring partial resistance) if the M.I.C. is 4 to 12.5 mcg/mL and resistant (not likely to respond to therapy) if the M.I.C. is greater than 12.5 mcg/mL. Susceptibility Plate Testing: If the Kirby-Bauer method of disc susceptibility is used, a 30 mcg doxycycline disc should give a zone of at least 16 mm when tested against a doxycycline-susceptible strain. A tetracycline disc may be used to determine microbial susceptibility. If the Kirby-Bauer method of disc susceptibility is used, a 30 mcg tetracycline disc should give a zone of at least 19 mm
when tested against a tetracycline-susceptible bacterial strain. Tetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations and in a biologically active form. Following a 100 mg single dose administered in a concentration of 0.4 mg/mL in a one-hour infusion, normal adult volunteers average a peak of 2.5 mcg/mL, while 200 mg of a concentration of 0.4 mg/mL administered over two hours average a peak of 3.6 mcg/mL. Excretion of doxycycline by the kidney is about 40 percent/72 hours in individuals with normal function (creatinine clearance about 75 mL/min). This percentage excretion may fall as low as 1 to 5 percent/72 hours in individuals with severe renal insufficiency creatinine clearance below 10 mL/min). Studies have shown no significant difference in serum half-life of doxycycline (range 18 to 22 hours) in individuals with normal and severely impaired renal function. Hemodialysis does not alter this serum half-life of doxycycline.
Doxycycline Indications and Usage
Doxycycline is indicated in infections caused by the following microorganisms:
• Rickettsiae (Rocky Mountain spotted fever, typhus fever, and the typhus group, Q fever, rickettsialpox and tick fevers),
• Mycoplasma pneumoniae (PPLO, Eaton Agent),
• Agents of psittacosis and ornithosis,
• Agents of lymphogranuloma venereum and granuloma inguinale,
• The spirochetal agent of relapsing fever (Borrelia recurrentis).
The following gram-negative microorganisms:
• Haemophilus ducreyi (chancroid),
• Pasteurella pestis and Pasteurella tularensis,
• Bartonella bacilliformis,
• Bacteroides species,
• Vibrio comma and Vibrio fetus,
• Brucella species (in conjunction with streptomycin).
Because many strains of the following groups of microorganisms have been shown to be resistant to tetracyclines, culture and susceptibility testing are recommended.
Doxycycline is indicated for treatment of infections caused by the following gram-negative microorganisms when bacteriologic testing indicates appropriate susceptibility to the drug:
• Escherichia coli,
• Enterobacter aerogenes (formerly Aerobacter aerogenes),
• Shigella species,
• Mima species and Herellea species,
• Haemophilus influenzae (respiratory infections),
• Klebsiella species (respiratory and urinary infections).
Doxycycline is indicated for treatment of infections caused by the following gram-positive microorganisms when bacteriologic testing indicates appropriate susceptibility to the drug:
• Streptococcus species: Up to 44 percent of strains of Streptococcus pyogenes and 74 percent of Streptococcus faecalis have been found to be resistant to tetracycline drugs. Therefore, tetracyclines should not be used for streptoccal disease unless the organism has been demonstrated to be sensitive.
• Anthrax due to Bacillus anthracis, including inhalational anthrax (post-exposure): to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis.
For upper respiratory infections due to group A beta-hemolytic streptococci, penicillin is the usual drug of choice, including prophylaxis of rheumatic fever.
• Diplococcus pneumoniae,
• Staphylococcus aureus, respiratory, skin and soft tissue infections.
Tetracyclines are not the drugs of choice in the treatment of any type of staphylococcal infections. When penicillin is contraindicated, doxycycline is an alternative drug in the treatment of infections due to:
• Neisseria gonorrhoeae and N. meningitis,
• Treponema pallidum and Treponema pertenue (syphilis and yaws),
• Listeria monocytogenes,
• Clostridium species,
• Fusobacterium fusiforme (Vincent's infection),
• Actinomyces species.
In acute intestinal amebiasis, doxycycline may be a useful adjunct to amebicides. Doxycycline is indicated in the treatment of trachoma, although the infectious agent is not always eliminated, as judged by immunofluorescence. To reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline and other antibacterial drugs,
doxycycline should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
The drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
Warnings
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN). TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED IN THIS AGE GROUP, EXCEPT FOR ANTHRAX, INCLUDING INHALATIONAL ANTHRAX, (POSTEXPOSURE), UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED.
This adverse reaction is more common during long-term use of the drugs but has been observed following repeated short-term courses. ENAMEL HYPOPLASIA HAS ALSO BEEN REPORTED. Tetracycline drugs, therefore, should not be used in this age group unless other drugs are not likely to be effective or are contraindicated. Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema. The antianabolic action of the tetracyclines may cause an increase in BUN. Studies to date indicate that this does not occur with the use of doxycycline in patients with impaired renal function.
Usage in Pregnancy
(See WARNINGS about use during tooth development).
Intravenous doxycycline has not been studied in pregnant patients. It should not be used in pregnant women unless, in the judgement of the physician, it is essential for the welfare of the patient. Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues and can have toxic effects on the developing fetus (often related to retardation of skeletal development). Evidence of embryotoxicity has also been noted in animals, treated early in pregnancy.
Pediatric Use
The use of doxycycline in pediatric patients under 8 years is not recommended because safe conditions for its use have not been established. (See WARNINGS about use during tooth development). As with other tetracyclines, doxycycline forms a stable calcium complex in any bone-forming tissue. A decrease in the fibula growth rate has been observed in prematures given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued. Tetracyclines are present in the milk of lactating women who are taking a drug in this class.
Precautions
General
Prescribing doxycycline in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. As with other antibiotic preparations, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, the antibiotic should be discontinued and appropriate therapy instituted. In venereal diseases when coexistent syphilis is suspected, a dark field examination should be done before treatment is started and the blood serology repeated monthly for at least 4 months. Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage. In long-term therapy, periodic laboratory evaluation of organ systems, including hematopoietic, renal and hepatic studies should be performed. All infections due to group A beta-hemolytic streptococci should be treated for at least 10 days. Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracycline in conjunction with penicillin.
Information for Patients
Patients should be counseled that antibacterial drugs including doxycycline should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When doxycycline is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by doxycycline or other antibacterial drugs in the future.
Side Effects
Gastrointestinal: anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, and inflammatory lesions (with monilial overgrowth) in the anogenital region. These reactions have been caused by both the oral and parenteral administration of tetracyclines. Skin: Maculopapular and erythematous rashes. Exfoliative dermatitis has been reported but is uncommon. Photosensitivity is discussed above. (See WARNINGS).
Renal Toxicity: Rise in BUN has been reported and is apparently dose related. (See WARNINGS). Hypersensitivity Reactions: urticaria, angioneurotic edema, anaphylaxis, anaphylactoid purpura, pericarditis and exacerbation of systemic lupus erythematosus. Bulging fontanels in infants and benign intracranial hypertension in adults have been reported in individuals receiving full therapeutic dosages. These conditions disappeared rapidly when the drug was discontinued.
Blood: Hemolytic anemia, thrombocytopenia, neutropenia and eosinophilia have been reported. When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function studies are known to occur.
Dosage and Administration
Note: Rapid administration is to be avoided. Parenteral therapy is indicated only when oral therapy is not indicated. Oral therapy should be instituted as soon as possible. If intravenous therapy is given over prolonged periods of time, thrombophlebitis may result. THE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF INTRAVENOUS DOXYCYCLINE (100 TO 200 MG/DAY) DIFFERS FROM THAT OF THE OTHER TETRACYCLINES (1 TO 2 G/DAY). EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS. Studies to date have indicated that doxycycline at the usual recommended doses does not lead to excessive accumulation of the antibiotic in patients with renal impairment.
Adults
The usual dosage of intravenous doxycycline is 200 mg on the first day of treatment administered in one or two infusions. Subsequent daily dosage is 100 to 200 mg depending upon the severity of infection, with 200 mg administered in one or two infusions. In the treatment of primary and secondary syphilis, the recommended dosage is 300 mg daily for at least 10 days.
In the treatment of inhalational anthrax (post-exposure) the recommended dose is 100 mg of doxycycline, twice a day. Parenteral therapy is only indicated when oral therapy is not indicated and should not be continued over a prolonged period of time. Oral therapy should be instituted as soon as possible. Therapy must continue for a total of 60 days.
For Children Above Eight Years of Age
The recommended dosage schedule for children weighing 100 pounds or less is 2 mg/lb of body weight on the first day of treatment, administered in one or two infusions. Subsequent daily dosage is 1 to 2 mg/lb of body weight given as one or two infusions, depending on the severity of the infection. For children over 100 pounds the usual adult dose should be used. (See WARNINGS: Pediatric Use). In the treatment of inhalational anthrax (post-exposure) the recommended dose is 1 mg/lb (2.2 mg/kg) of body weight, twice a day in the children weighing less than 100 lb (45 kg). Parenteral therapy is only indicated when oral therapy is not indicated and should not be continued over a prolonged period of time. Oral therapy should be instituted as soon as possible. Therapy must continue for a total of 60 days.
General
The duration of infusion may vary with the dose (100 to 200 mg per day), but is usually one to four hours. A recommended minimum infusion time for 100 mg of a 0.5 mg/mL solution is one hour. Therapy should be continued for at least 24 to 48 hours after symptoms and fever have subsided. The therapeutic antibacterial serum activity will usually persist for 24 hours following recommended dosage. Intravenous solutions should not be injected intramuscularly or subcutaneously. Caution should be taken to avoid the inadvertent introduction of the intravenous solution into the adjacent soft tissue.
Preparation of Solution
To prepare a solution containing 10 mg/mL, the contents of the vial should be reconstituted with 10 mL of Sterile Water for Injection, or any of the ten intravenous infusion solutions listed below. Each 100 mg of doxycycline (i.e., withdraw entire solution from the 100 mg vial) is further diluted with 100 to 1000 mL of the intravenous solutions listed below:
1. 0.9% Sodium Chloride Injection
2. 5% Dextrose Injection
3. Ringer's Injection
4. Invert Sugar, 10% in Water
5. Lactated Ringer's Injection
6. Dextrose 5% in Lactated Ringer's
7. Normosol-M® in D5-W (Abbott)
8. Normosol-R® in D5-W (Abbott)
9. Plasma-Lyte® 56 in 5% Dextrose (Travenol)
10. Plasma-Lyte® 148 in 5% Dextrose (Travenol)
This will result in desired concentrations of 0.1 to 1 mg/mL. Concentrations lower than 0.1 mg/mL or higher than 1 mg/mL are not recommended.
Stability
Doxycycline is stable for 48 hours in solution when diluted with Sodium Chloride Injection, or 5% Dextrose Injection, to concentrations between 1 mg/mL and 0.1 mg/mL and stored at 25°C. Doxycycline in these solutions is stable under fluorescent light for 48 hours, but must be protected from direct sunlight during storage and infusion. Reconstituted solutions (1 to 0.1 mg/mL) may be stored up to 72 hours prior to start of infusion if refrigerated and protected from sunlight and artificial light. Infusion must then be completed within 12 hours. Solutions must be used within these time periods or discarded. Doxycycline, when diluted with Ringer's Injection, or Invert Sugar, 10% in Water, or Normosol-M® in D5-W (Abbott), or Normosol- R® in D5-W (Abbott), or Plasma-Lyte® 56 in 5% Dextrose (Travenol), or Plasma-Lyte® 148 in 5% Dextrose (Travenol) to a concentration between 1 mg/mL and 0.1 mg/mL, must be completely infused within 12 hours after reconstitution to ensure adequate stability. During infusion, the solution must be protected from direct sunlight. Reconstituted solutions (1 to 0.1 mg/mL) may be stored up to 72 hours prior to start of infusion, if refrigerated and protected from sunlight and artificial light. Infusion must then be completed within 12 hours. Solutions must be used within these time periods or discarded. When diluted with Lactated Ringer's Injection, or Dextrose 5% in Lactated Ringer's, infusion of the solution (ca. 1 mg/mL) or lower concentrations (not less than 0.1 mg/mL) must be completed within six hours after reconstitution to ensure adequate stability. During infusion, the solution must be protected from direct sunlight. Solutions must be used within this time period or discarded. Solutions of doxycycline hyclate at a concentration of 10 mg/mL in Sterile Water for Injection, when frozen immediately after reconstitution are stable for 8 weeks when stored at –20°C. If the product is warmed, care should be taken to avoid heating it after the thawing is complete. Once thawed the solution should not be refrozen. Parenteral drug products should be inspected visually for particulate matter and discoloration whenever solution and container permit.
How Supplied
Doxycycline for Injection USP is supplied as a sterile lyophilized powder in a single-use vial. Retain in carton until time of use. NDC 55390-110-10 Each vial contains doxycycline hyclate equivalent to 100 mg doxycycline and 480 mg ascorbic acid. 10 vials per carton.
Store lyophilized product at or below 25°C (77°F), and protect from light.
Manufactured by: Manufactured for:
Ben Venue Laboratories, Inc. Bedford Laboratories™
Bedford, OH 44146 Bedford, OH 44146
September 2004 Div-DCY-P03
Sample Outer Label

DoxycyclineDoxycycline INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||