Doxazosin
Bryant Ranch Prepack
Bryant Ranch Prepack
DOXAZOSIN TABLETS USP 8120 8121 8122 8123 Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- DOXAZOSIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- DOXAZOSIN INDICATIONS AND USAGE
- DOXAZOSIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DOXAZOSIN ADVERSE REACTIONS
- OVERDOSAGE
- DOXAZOSIN DOSAGE AND ADMINISTRATION
- PATIENT INFORMATION
- Doxazosin Mesylate 4mg Tablet
FULL PRESCRIBING INFORMATION
DOXAZOSIN DESCRIPTION
Doxazosin mesylate is a quinazoline compound that is a selective inhibitor of the alpha subtype of alpha-adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate. It has the following structure: 1
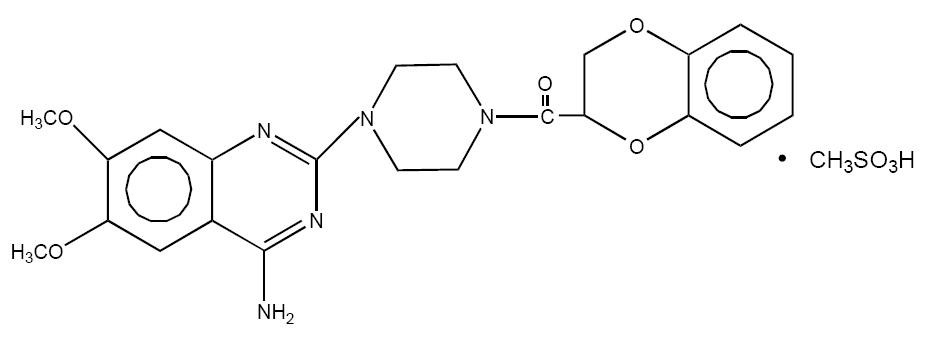
C H N O •CH O S M.W. 547.6 23 25 5 5 4 3
Doxazosin mesylate is freely soluble in dimethylsulfoxide, soluble in dimethylformamide, slightly soluble in methanol, ethanol, and water (0.8% at 25°C), and very slightly soluble in acetone and methylene chloride. Each doxazosin tablet USP, for oral administration, contains 1 mg, 2 mg, 4 mg, or 8 mg of doxazosin as the free base.
The inactive ingredients for all tablets are: microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, magnesium stearate, pregelatinized starch, and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
DOXAZOSIN INDICATIONS AND USAGE
DOXAZOSIN CONTRAINDICATIONS
Doxazosin mesylate is contraindicated in patients with a known sensitivity to quinazolines (e.g., prazosin, terazosin), doxazosin, or any of the inert ingredients.
WARNINGS
PRECAUTIONS
General
Information for Patients (See Patient Package Insert)
Patients should be made aware of the possibility of syncopal and orthostatic symptoms, especially at the initiation of therapy, and urged to avoid driving or hazardous tasks for 24 hours after the first dose, after a dosage increase, and after interruption of therapy when treatment is resumed. They should be cautioned to avoid situations where injury could result should syncope occur during initiation of doxazosin therapy. They should also be advised of the need to sit or lie down when symptoms of lowered blood pressure occur, although these symptoms are not always orthostatic, and to be careful when rising from a sitting or lying position. If dizziness, lightheadedness, or palpitations are bothersome, they should be reported to the physician, so that dose adjustment can be considered. Patients should also be told that drowsiness or somnolence can occur with doxazosin mesylate or any selective alpha adrenoceptor antagonist, requiring caution in people who must drive or operate heavy machinery. 1
Patients should be advised about the possibility of priapism as a result of treatment with alpha antagonists. Patients should know that this adverse event is very rare. If they experience priapism, it should be brought to immediate medical attention, for, if not treated promptly, it can lead to permanent erectile dysfunction (impotence). 1
Drug/Laboratory Test Interactions
Doxazosin mesylate does not affect the plasma concentration of prostate-specific antigen in patients treated for up to 3 years. Both doxazosin, an alpha inhibitor, and finasteride, a 5-alpha reductase inhibitor, are highly protein-bound and hepatically metabolized. There is no definitive controlled clinical experience on the concomitant use of alpha inhibitors and 5-alpha reductase inhibitors at this time. 1 1
Drug Interactions
Most (98%) of plasma doxazosin is protein bound. data in human plasma indicate that doxazosin mesylate has no effect on protein binding of digoxin, warfarin, phenytoin, or indomethacin. There is no information on the effect of other highly plasma protein-bound drugs on doxazosin binding. Doxazosin mesylate has been administered without any evidence of an adverse drug interaction to patients receiving thiazide diuretics, beta-blocking agents, and non-steroidal anti-inflammatory drugs. In a placebo-controlled trial in normal volunteers, the administration of a single 1 mg dose of doxazosin on day 1 of a four-day regimen of oral cimetidine (400 mg twice daily) resulted in a 10% increase in mean AUC of doxazosin (p = 0.006), and a slight but not statistically significant increase in mean C and mean half-life of doxazosin. The clinical significance of this increase in doxazosin AUC is unknown. In vitro max
In clinical trials, doxazosin mesylate tablets have been administered to patients on a variety of concomitant medications; while no formal interaction studies have been conducted, no interactions were observed. Doxazosin mesylate tablets have been used with the following drugs or drug classes: 1) analgesic/anti-inflammatory (e.g., acetaminophen, aspirin, codeine and codeine combinations, ibuprofen, indomethacin); 2) antibiotics (e.g., erythromycin, trimethoprim and sulfamethoxazole, amoxicillin); 3) antihistamines (e.g., chlorpheniramine); 4) cardiovascular agents (e.g., atenolol, hydrochlorothiazide, propranolol); 5) corticosteroids; 6) gastrointestinal agents (e.g., antacids); 7) hypoglycemics and endocrine drugs; 8) sedatives and tranquilizers (e.g., diazepam); 9) cold and flu remedies.
Concomitant administration of doxazosin mesylate with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension (see ). DOSAGE AND ADMINISTRATION
Carcinogenesis, Mutagenesis, Impairment of Fertility
Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses of 40 mg/kg/day in rats and 120 mg/kg/day in mice revealed no evidence of carcinogenic potential. The highest doses evaluated in the rat and mouse studies are associated with AUCs (a measure of systemic exposure) that are 8 times and 4 times, respectively, the human AUC at a dose of 16 mg/day.
Mutagenicity studies revealed no drug- or metabolite-related effects at either chromosomal or subchromosomal levels.
Studies in rats showed reduced fertility in males treated with doxazosin at oral doses of 20 (but not 5 or 10) mg/kg/day, about 4 times the AUC exposures obtained with a 12 mg/day human dose. This effect was reversible within two weeks of drug withdrawal. There have been no reports of any effects of doxazosin on male fertility in humans.
Pregnancy
Teratogenic Effects
Nonteratogenic Effects
In peri-postnatal studies in rats, postnatal development at maternal doses of 40 or 50 mg/kg/day of doxazosin (8 times human AUC exposure with a 12 mg/day therapeutic dose) was delayed, as evidenced by slower body weight gain and slightly later appearance of anatomical features and reflexes.
Nursing Mothers
Studies in lactating rats given a single oral dose of 1 mg/kg of [2- C]-doxazosin mesylate indicate that doxazosin accumulates in rat breast milk with a maximum concentration about 20 times greater than the maternal plasma concentration. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doxazosin mesylate is administered to a nursing mother. 14
Pediatric Use
The safety and effectiveness of doxazosin mesylate as an antihypertensive agent have not been established in children.
Geriatric Use
The safety and effectiveness profile of doxazosin mesylate in BPH was similar in the elderly (age ≥ 65 years) and younger (age < 65 years) patients.
DOXAZOSIN ADVERSE REACTIONS
OVERDOSAGE
Experience with doxazosin mesylate overdosage is limited. Two adolescents, who each intentionally ingested 40 mg doxazosin mesylate with diclofenac or acetaminophen, were treated with gastric lavage with activated charcoal and made full recoveries. A two-year-old child who accidently ingested 4 mg doxazosin mesylate was treated with gastric lavage and remained normotensive during the five-hour emergency room observation period. A six-month-old child accidentally received a crushed 1 mg tablet of doxazosin mesylate and was reported to have been drowsy. A 32-year-old female with chronic renal failure, epilepsy, and depression intentionally ingested 60 mg doxazosin mesylate (blood level = 0.9 mcg/mL; normal values in hypertensives = 0.02 mcg/mL); death was attributed to a grand mal seizure resulting from hypotension. A 39-year-old female who ingested 70 mg doxazosin mesylate, alcohol, and Dalmane (flurazepam) developed hypotension which responded to fluid therapy. ®
The oral LD of doxazosin is greater than 1000 mg/kg in mice and rats. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of fluid. As doxazosin is highly protein bound, dialysis would not be indicated. 50
DOXAZOSIN DOSAGE AND ADMINISTRATION
The initial dosage of doxazosin tablets USP in patients with hypertension and/or BPH is 1 mg given once daily in the a.m. or p.m. This starting dose is intended to minimize the frequency of postural hypotension and first-dose syncope associated with doxazosin tablets USP. Postural effects are most likely to occur between 2 and 6 hours after a dose. Therefore, blood pressure measurements should be taken during this time period after the first dose and with each increase in dose. If doxazosin tablet USP administration is discontinued for several days, therapy should be restarted using the initial dosing regimen. DOSAGE MUST BE INDIVIDUALIZED .
Concomitant administration of doxazosin tablets USP with a PDE-5 inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension; therefore, PDE-5 inhibitor therapy should be initiated at the lowest dose in patients taking doxazosin tablets USP.
PATIENT INFORMATION
ABOUT DOXAZOSIN TABLETS USP FOR BENIGN PROSTATIC HYPERPLASIA (BPH)
Rx only
Read this leaflet:
- before you start taking doxazosin tablets USP
- each time you get a new prescription
You and your doctor should discuss this treatment and your BPH symptoms before you start taking doxazosin tablets USP and at your regular checkups. This leaflet does NOT take the place of discussions with your doctor.
Doxazosin tablets USP are used to treat both benign prostatic hyperplasia (BPH) and high blood pressure (hypertension). This leaflet describes doxazosin tablets USP as treatment for BPH (although you may be taking doxazosin tablets USP for both your BPH and high blood pressure).
What is BPH?
BPH is an enlargement of the prostate gland. This gland surrounds the tube that drains the urine from the bladder. The symptoms of BPH can be caused by a tensing of the enlarged muscle in the prostate gland which blocks the passage of urine. This can lead to such symptoms as:
- a weak or start-and-stop stream when urinating
- a feeling that the bladder is not completely emptied after urination
- a delay or difficulty in the beginning of urination
- a need to urinate often during the day and especially at night
- a feeling that you must urinate immediately
Treatment Options for BPH
The four main treatment options for BPH are:
- If you are not bothered by your symptoms, you and your doctor may decide on a program of “watchful waiting.” It is not an active treatment like taking medication or surgery but involves having regular checkups to see if your condition is getting worse or causing problems.
- Treatment with doxazosin tablets USP or other similar drugs. Doxazosin tablets USP are the medication your doctor has prescribed for you. See “ ,” below. What Doxazosin Tablets USP Do
- Treatment with the medication class of 5-alpha reductase inhibitors (e.g., Proscar ). It can cause the prostate to shrink. It may take 6 months or more for the full benefit of finasteride to be seen. ®
- Various surgical procedures. Your doctor can describe these procedures to you. The best procedure for you depends on your BPH symptoms and medical condition.
What Doxazosin Tablets USP Do
Doxazosin tablets USP work on a specific type of muscle found in the prostate, causing it to relax. This in turn decreases the pressure within the prostate, thus improving the flow of urine and your symptoms.
- Doxazosin tablets USP help relieve the symptoms of BPH (weak stream, start-and-stop stream, a feeling that your bladder is not completely empty, delay in beginning of urination, need to urinate often during the day and especially at night, and feeling that you must urinate immediately). It does not change the size of the prostate. The prostate may continue to grow; however, a larger prostate is not necessarily related to more symptoms or to worse symptoms. Doxazosin tablets USP can decrease your symptoms and improve urinary flow, without decreasing the size of the prostate.
- If doxazosin tablets USP are helping you, you should notice an effect within 1 to 2 weeks after you start your medication. Doxazosin tablets USP have been studied in over 900 patients for up to 2 years and the drug has been shown to continue to work during long-term treatment. Even though you take doxazosin tablets USP and they may help you, doxazosin tablets USP may not prevent the need for surgery in the future.
- Doxazosin tablets USP do not affect PSA levels. PSA is the abbreviation for Prostate Specific Antigen. Your doctor may have done a blood test called PSA. You may want to ask your doctor more about this if you have had a PSA test done.
Other Important Facts
- You should see an improvement of your symptoms within 1 to 2 weeks. In addition to your other regular checkups you will need to continue seeing your doctor regularly to check your progress regarding your BPH and to monitor your blood pressure.
- Doxazosin tablets USP are not a treatment for prostate cancer. Your doctor has prescribed doxazosin tablets USP for your BPH and not for prostate cancer; however, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). A higher incidence of prostate cancer has been noted in men of African-American descent. These checks should continue even if you are taking doxazosin tablets USP.
How To Take Doxazosin Tablets USP and What You Should Know While Taking Doxazosin Tablets USP for BPH
You may feel dizzy, faint or “light-headed,” especially after you stand up from a lying or sitting position. This is more likely to occur after you’ve taken the first few doses or if you increase your dose, but can occur at any time while you are taking the drug. It can also occur if you stop taking the drug and then restart treatment. If you feel very dizzy, faint or “light-headed” you should contact your doctor. Your doctor will discuss with you how often you need to visit and how often your blood pressure should be checked. Doxazosin Tablets USP Can Cause a Sudden Drop in Blood Pressure After the VERY FIRST DOSE.
Your blood pressure should be checked when you start taking doxazosin tablets USP even if you do not have high blood pressure (hypertension). Your doctor will discuss with you the details of how blood pressure is measured.
Whatever equipment is used, it is usual for your blood pressure to be measured in the following way: measure your blood pressure after lying quietly on your back for five minutes. Then, after standing for two minutes measure your blood pressure again. Your doctor will discuss with you what other times during the day your blood pressure should be taken, such as two to six hours after a dose, before bedtime or after waking up in the morning. Note that moderate to high-intensity exercise can, over a period of time, lower your average blood pressure. Blood Pressure Measurement :
You can take doxazosin tablets USP either in the morning or at bedtime and it will be equally effective. If you take doxazosin tablets USP at bedtime but need to get up from bed to go to the bathroom, get up slowly and cautiously until you are sure how the medication affects you. It is important to get up slowly from a chair or bed at any time until you learn how you react to doxazosin tablets USP. You should not drive or do any hazardous tasks until you are used to the effects of the medication. If you begin to feel dizzy, sit or lie down until you feel better.
- You will start with a 1 mg dose of doxazosin tablets USP once daily. Then the once daily dose will be increased as your body gets used to the effects of the medication. Follow your doctor’s instructions about how to take doxazosin tablets USP. You must take it every day at the dose prescribed. Talk with your doctor if you don’t take it for a few days for some reason; you may then need to restart the medication at a 1 mg dose, increase your dose gradually and again be cautious about possible dizziness. Do not share doxazosin tablets USP with anyone else; they were prescribed only for you.
- Other side effects you could have while taking doxazosin tablets USP, in addition to lowering of the blood pressure, include dizziness, fatigue (tiredness), swelling of the feet and shortness of breath. Most side effects are mild. However, you should discuss any unexpected effects you notice with your doctor.
- Extremely rarely, doxazosin tablets USP and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible. WARNING:
- Tell your surgeon if you take or have taken doxazosin tablets USP if you plan to have surgery for cataracts (clouding of the eye). During cataract surgery, a condition called Intraoperative Floppy Iris Syndrome (IFIS) can happen if you take or have taken doxazosin tablets USP.
- If you use doxazosin tablets USP with an oral erectile dysfunction medicine (phosphodiesterase-5 (PDE-5) inhibitor), it can cause a sudden drop in your blood pressure and you can become dizzy or faint. Talk with your healthcare provider before using PDE-5 inhibitors.
- Keep doxazosin tablets USP and all medicines out of the reach of children.
FOR MORE INFORMATION ABOUT DOXAZOSIN TABLETS USP AND BPH TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Teva Pharmaceuticals USA.
Manufactured In Canada By:
NOVOPHARM LIMITED
Toronto, Canada M1B 2K9
Manufactured For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. B 3/2010
Doxazosin Mesylate 4mg Tablet

DoxazosinDoxazosin Mesylate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||