Donepezil Hydrochloride
Sun Pharmaceutical Industries Limited
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use donepezil hydrochloride safely and effectively. See full prescribing information for donepezil hydrochloride tablets Donepezil Hydrochloride TabletsInitial U.S. Approval: 1996 INDICATIONS AND USAGE1DOSAGE AND ADMINISTRATION Mild to Moderate Alzheimer’s Disease - 5 mg or 10 mg administered once daily (2.1) Severe Alzheimer’s Disease - 10 mg administered once daily (2.2) 2.3DOSAGE FORMS AND STRENGTHS Tablets: 5 mg and 10 mg (3) CONTRAINDICATIONS Patients with known hypersensitivity to donepezil hydrochloride or to piperidine derivatives (4) WARNINGS AND PRECAUTIONS Cholinesterase inhibitors are likely to exaggerate succinylcholine-type muscle relaxation during anesthesia (5.1). Cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes manifesting as bradycardia or heart block (5.2). Donepezil hydrochloride can cause vomiting. Patients should be observed closely at initiation of treatment and after dose increases (5.3). Patients should be monitored closely for symptoms of active or occult gastrointestinal (GI) bleeding, especially those at increased risk for developing ulcers (5.4). Cholinomimetics may cause bladder outflow obstructions (5.6). Cholinomimetics are believed to have some potential to cause generalized convulsions (5.7). Cholinesterase inhibitors should be prescribed with care to patients with a history of asthma or obstructive pulmonary disease (5.8). Side Effects6.1To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Cholinesterase inhibitors have the potential to interfere with the activity of anticholinergic medications (7.3). A synergistic effect may be expected with concomitant administration of succinylcholine, similar neuromuscular blocking agents, or cholinergic agonists (7.4). USE IN SPECIFIC POPULATIONS Based on animal data, donepezil hydrochloride may cause fetal harm (8.1).
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 DONEPEZIL HYDROCHLORIDE INDICATIONS AND USAGE
- 2 DONEPEZIL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 DONEPEZIL HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 DONEPEZIL HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DONEPEZIL HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 5MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 5MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 10MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 10MG
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1. Mild to Moderate Alzheimer's Disease
2.2. Severe Alzheimer's Disease
2.3. Titration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1. Anesthesia
5.2. Cardiovascular Conditions
5.3. Nausea and Vomiting
5.4. Peptic Ulcer Disease and GI Bleeding
5.6 Genitourinary Conditions
5.7 Neurological Conditions: Seizures
5.8 Pulmonary Conditions
6 ADVERSE REACTIONS
6.1. Clinical Studies Experience
Mild to Moderate Alzheimer’s Disease
Adverse Events Leading to Discontinuation
| Dose Group | Placebo | 5 mg/day Donepezil Hydrochloride | 10 mg/day Donepezil Hydrochloride |
|---|---|---|---|
|
Patients Randomized
|
355 |
350 |
315 |
|
Event/%Discontinuing
|
|
|
|
| Nausea |
1% |
1% |
3% |
| Diarrhea |
0% |
<1% |
3% |
| Vomiting |
<1% |
<1% |
2% |
| No titration | One week titration | Six week titration | ||
|---|---|---|---|---|
| Adverse Event | Placebo (n=315) | 5 mg/day (n=311) | 10 mg/day (n=315) | 10 mg/day (n=269) |
| Nausea |
6% |
5% |
19% |
6% |
| Diarrhea |
5% |
8% |
15% |
9% |
| Insomnia |
6% |
6% |
14% |
6% |
| Fatigue |
3% |
4% |
8% |
3% |
| Vomiting |
3% |
3% |
8% |
5% |
| Muscle cramps |
2% |
6% |
8% |
3% |
| Anorexia |
2% |
3% |
7% |
3% |
| Body System/Adverse Event | Placebo (n=355) |
Donepezil Hydrochloride (n=747) |
|---|---|---|
|
Percent of Patients with any Adverse Event
|
72
|
74
|
|
Body as a Whole
|
||
| Headache |
9 |
10 |
| Pain, various locations |
8 |
9 |
| Accident |
6 |
7 |
| Fatigue |
3 |
5 |
|
Cardiovascular System
|
||
| Syncope |
1 |
2 |
|
Digestive System
|
||
| Nausea |
6 |
11 |
| Diarrhea |
5 |
10 |
| Vomiting |
3 |
5 |
| Anorexia |
2 |
4 |
|
Hemic and Lymphatic System
|
||
| Ecchymosis |
3 |
4 |
|
Metabolic and Nutritional Systems
|
||
| Weight Decrease |
1 |
3 |
|
Musculoskeletal System
|
||
| Muscle Cramps |
2 |
6 |
| Arthritis |
1 |
2 |
|
Nervous System
|
||
| Insomnia |
6 |
9 |
| Dizziness |
6 |
8 |
| Depression |
<1 |
3 |
| Abnormal Dreams |
0 |
3 |
| Somnolence |
<1 |
2 |
|
Urogenital System
|
||
| Frequent Urination |
1 |
2 |
Frequent adverse eventsInfrequent adverse events
Body as a Whole: Frequent: Infrequent:
Cardiovascular System: Frequent: Infrequent:
Digestive System: Frequent: Infrequent:
Endocrine System: Infrequent:
Hemic and Lymphatic System: Infrequent:
Metabolic and Nutritional Disorders: Frequent: Infrequent:
Musculoskeletal System: Frequent: Infrequent:
Nervous System: Frequent: Infrequent:
Respiratory System: Frequent: Infrequent:
Skin and Appendages: Frequent: Infrequent:
Special Senses: Frequent: Infrequent:
Urogenital System: Frequent: Infrequent:
Severe Alzheimer’s Disease
Adverse Events Leading to Discontinuation
Most Frequent Adverse Events Seen in Association with the Use of Donepezil Hydrochloride
Adverse Events Reported in Controlled Trials
| Body System/Adverse Event | Placebo (n=392) |
Donepezil Hydrochloride(n=501) |
|---|---|---|
|
Percent of Patients with any Adverse Event
|
73
|
81
|
|
Body as a Whole
|
||
| Accident |
12 |
13 |
| Infection |
9 |
11 |
| Headache |
3 |
4 |
| Pain |
2 |
3 |
| Back Pain |
2 |
3 |
| Fever |
1 |
2 |
| Chest Pain |
<1 |
2 |
|
Cardiovascular System
|
||
| Hypertension |
2 |
3 |
| Hemorrhage |
1 |
2 |
| Syncope |
1 |
2 |
|
Digestive System
|
||
| Diarrhea |
4 |
10 |
| Vomiting |
4 |
8 |
| Anorexia |
4 |
8 |
| Nausea |
2 |
6 |
|
Hemic and Lymphatic System
|
||
| Ecchymosis |
2 |
5 |
|
Metabolic and Nutritional Systems
|
||
| Creatine Phosphokinase Increased |
1 |
3 |
| Dehydration |
1 |
2 |
| Hyperlipemia |
<1 |
2 |
|
Nervous System
|
||
| Insomnia |
4 |
5 |
| Hostility |
2 |
3 |
| Nervousness |
2 |
3 |
| Hallucinations |
1 |
3 |
| Somnolence |
1 |
2 |
| Dizziness |
1 |
2 |
| Depression |
1 |
2 |
| Confusion |
1 |
2 |
| Emotional Lability |
1 |
2 |
| Personality Disorder |
1 |
2 |
|
Skin And Appendages
|
||
| Eczema |
2 |
3 |
|
Urogenital System
|
||
| Urinary Incontinence |
1 |
2 |
Frequent adverse events Infrequent adverse events
Body as a Whole: Frequent: Infrequent:
Cardiovascular System: Frequent: Infrequent:
Digestive System: Frequent: Infrequent:
Endocrine System: Infrequent: Hemic and Lymphatic System: Frequent: Infrequent
Metabolic and Nutritional Disorders: Frequent: Infrequent
Musculoskeletal System: Frequent: Infrequent:
Nervous System: Frequent: Infrequent:
Respiratory System: Frequent: Infrequent:
Skin and Appendages: Frequent: Infrequent:
Special Senses: Infrequent:
Urogenital System: Frequent: Infrequent:
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1. Effect of Donepezil Hydrochloride on the Metabolism of Other Drugs
in vivo in vitro
7.2. Effect of Other Drugs on the Metabolism of Donepezil Hydrochloride
in vitro0-24max
7.3. Use with Anticholinergics
7.4. Use with Cholinomimetics and Other Cholinesterase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C:
222
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
Because strategies for the management of overdose are continually evolving, it is advisable to contact a Poison Control Center to determine the latest recommendations for the management of an overdose of any drug.
11 DESCRIPTION
H24293
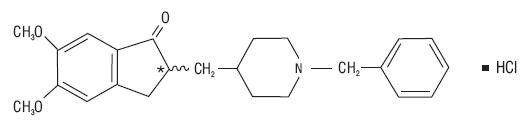 Donepezil hydrochloride
Donepezil hydrochloride12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
1
14in vitro
Hepatic Disease
Renal Disease2
Age:
Gender and Race:
Drugs Highly Bound to Plasma Proteinsin vitro
13 NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
22
in vitro in vitro tkin vitro in vivo
2
13.2. Animal Toxicology and/or pharmacology
14 CLINICAL STUDIES
14.1 Mild to Moderate Alzheimer’s Disease
Study Outcome Measures:
Thirty-Week Study
Effects on the ADAS-cog:
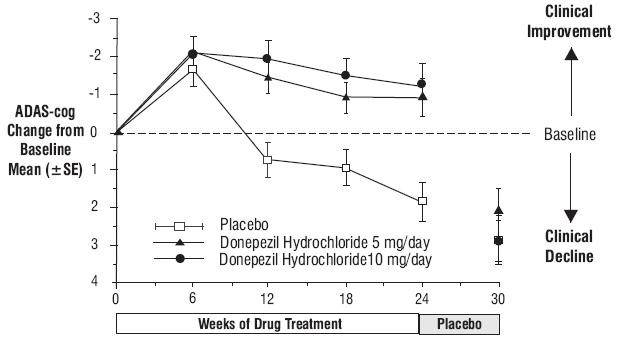 Figure 1. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing 24 Weeks of Treatment
Figure 1. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing 24 Weeks of Treatment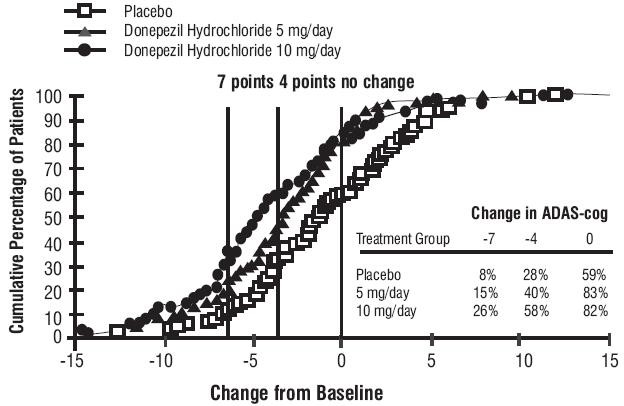 Figure 2. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients who Completed the Study were : Placebo 80%, 5 mg/day 85% and 10 mg/day 68%.
Figure 2. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients who Completed the Study were : Placebo 80%, 5 mg/day 85% and 10 mg/day 68%.
Effects on the CIBIC-plus:
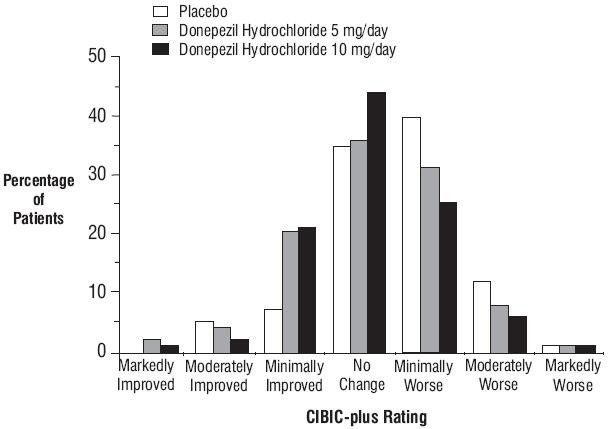 Figure 3. Frequency Distribution of CIBIC plus Scores at Week 24
Figure 3. Frequency Distribution of CIBIC plus Scores at Week 24
Fifteen-Week Study
Effects on the ADAS-Cog:
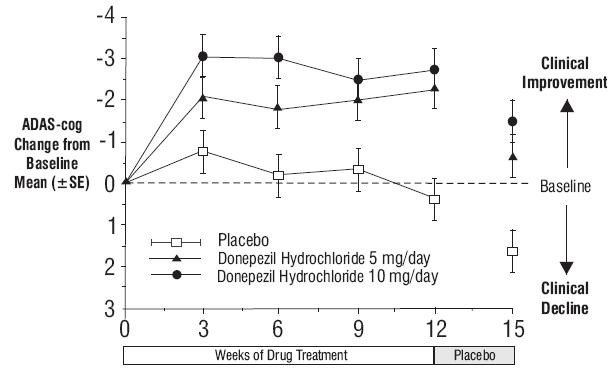 Figure 4. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing the 15-week Study.
Figure 4. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing the 15-week Study.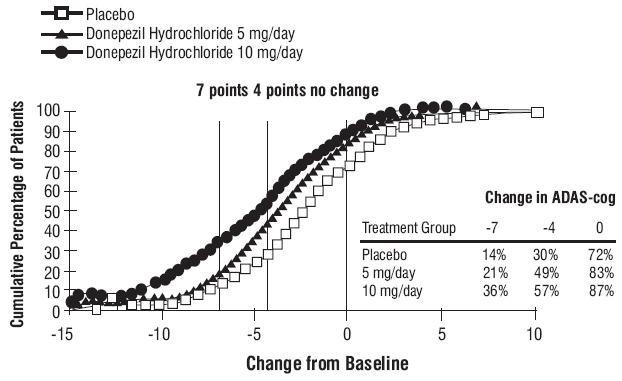 Figure 5. Cumulative Percentage of Patients with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients Within Each Treatment Group Who Completed the Study Were: Placebo 93%, 5 mg/day 90% and 10 mg/day 82%.
Figure 5. Cumulative Percentage of Patients with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients Within Each Treatment Group Who Completed the Study Were: Placebo 93%, 5 mg/day 90% and 10 mg/day 82%.
Effects on the CIBIC-plus:
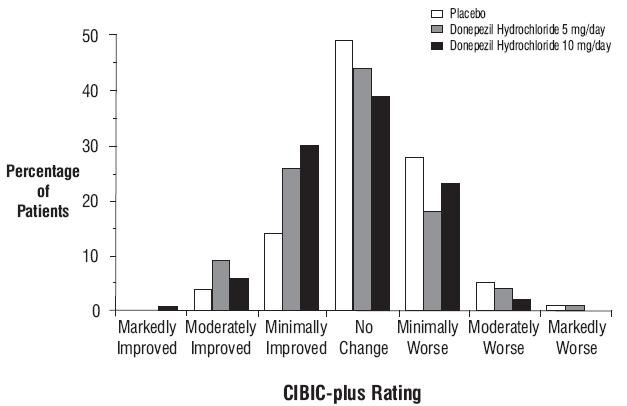 Figure 6. Frequency Distribution of CIBIC plus Scores at Week 12
Figure 6. Frequency Distribution of CIBIC plus Scores at Week 1214.2 Severe Alzheimer’s Disease
Swedish 6 Month Study
Study Outcome Measures:
Effects on the SIB:
 Figure 7. Time Course of the Change from Baseline in SIB Score for Patients Completing 6 months of Treatment
Figure 7. Time Course of the Change from Baseline in SIB Score for Patients Completing 6 months of Treatment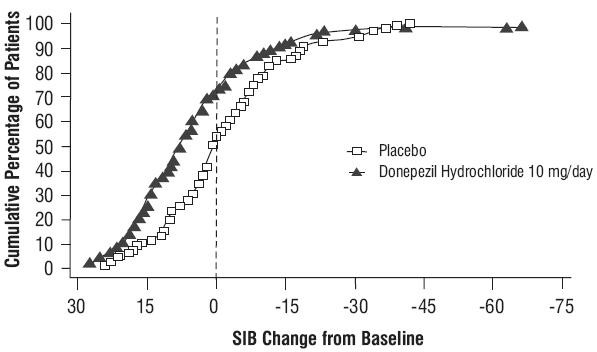 Figure 8. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in SIB Scores.
Figure 8. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in SIB Scores.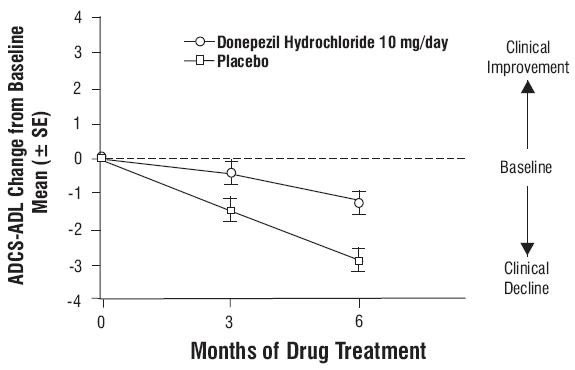 Figure 9. Time Course of the Change from Baseline in ADCS-ADL-Severe Score for Patients Completing 6 Months of Treatment
Figure 9. Time Course of the Change from Baseline in ADCS-ADL-Severe Score for Patients Completing 6 Months of Treatment
Effects on the ADCS-ADL-severe:
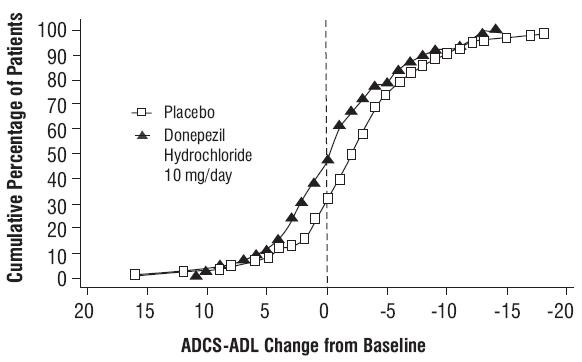 Figure 10. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in ADCS-ADL- Severe Scores.
Figure 10. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in ADCS-ADL- Severe Scores.
Japanese 24-Week Study
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1. Donepezil Hydrochloride Tablets
Storage:
17 PATIENT COUNSELING INFORMATION
PATIENT PACKAGE INSERT
Donepezil Hydrochloride Tablets
5 mg and 10 mg
What are donepezil hydrochloride tablets?
- Seem much better
- Get better in small ways or stay the same
- Get worse over time but slower than expected
- Not change and then get worse as expected
Who should not take donepezil hydrochloride tablets?
What should I tell the doctor before the patient takes donepezil hydrochloride tablets?
Tell the doctor about all the patient’s present or past health problems.
- Any heart problems including problems with irregular, slow, or fast heartbeats
- Asthma or lung problems
- A seizure
- Stomach ulcers
- Difficulty passing urine
- Liver or kidney problems
- Trouble swallowing tablets
- Present pregnancy or plans to become pregnant. It is not known if donepezil hydrochloride tablets can harm an unborn baby.
- Present breast-feeding. It is not known if donepezil hydrochloride passes into breast milk. Donepezil hydrochloride tablets are not for women who are breast-feeding.
- surgery
- medical procedures
- dental surgery or procedures.
How should the patient take donepezil hydrochloride tablets?
- Give donepezil hydrochloride tablets exactly as prescribed by the doctor. Do not stop donepezil hydrochloride tablets or change the dose yourself. Talk with the doctor first.
- Give donepezil hydrochloride tablets one time each day. Donepezil hydrochloride tablets can be taken with or without food.
- If you miss giving the patient a dose of donepezil hydrochloride tablets, just wait. Give only the next dose at the usual time. Do not give 2 doses at the same time.
- If donepezil hydrochloride tablets are missed for 7 days or more, talk with the doctor before starting again.
- If the patient takes too many donepezil hydrochloride tablets at one time, call the doctor or poison control center, or go to the emergency room right away.
Donepezil hydrochloride tablets may cause the following serious side effects:
- slow heartbeat and fainting. This happens more often in people with heart problems. Call the doctor right away if the patient faints while taking donepezil hydrochloride tablets.
- more stomach acid. This raises the chance of ulcers and bleeding. The risk is higher for patients who had ulcers, or take aspirin or other NSAIDs.
- worsening of lung problems in people with asthma or other lung disease.
- seizures.
- difficulty passing urine.
Call the doctor right away if the patient has:
- fainting.
- heartburn or stomach pain that is new or won’t go away.
- nausea or vomiting, blood in the vomit, dark vomit that looks like coffee grounds.
- bowel movements or stools that look like black tar.
- new or worse asthma or breathing problems.
- seizures.
- difficulty passing urine.
The most common side effects of donepezil hydrochloride tablets are:
- nausea
- diarrhea
- not sleeping well
- vomiting
- muscle cramps
- feeling tired
- not wanting to eat
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should donepezil hydrochloride tablets be stored?
Keep donepezil hydrochloride tablets and all medicines out of the reach of children.
General information about donepezil hydrochloride tablets
What are the ingredients in donepezil hydrochloride tablets?
Active ingredient:
Inactive ingredients:
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Industries Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 5MG
NDC 62756-440-81
Donepezil Hydrochloride Tablets
5 mg
Rx only
90 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
Pharmacist: Dispense the Patient information sheet

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 5MG
NDC 62756-440-65
Donepezil Hydrochloride Tablets
5 mg
Rx only
For in-institution use only
100 (10 X 10) Unit-Dose Tablets
THIS UNIT-DOSE PACKAGE IS NOT CHILD-RESISTANT
SUN PHARMACEUTICAL INDUSTRIES LTD.
Pharmacist: Dispense the Patient information sheet

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 10MG
NDC 62756-445-81
Donepezil Hydrochloride Tablets
10 mg
Rx only
90 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
Pharmacist: Dispense the Patient information sheet

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - CARTON - 10MG
NDC 62756-445-65
Donepezil Hydrochloride Tablets
10 mg
Rx only
For in-institution use only
100 (10 X 10) Unit-Dose Tablets
THIS UNIT-DOSE PACKAGE IS NOT CHILD-RESISTANT
SUN PHARMACEUTICAL INDUSTRIES LTD.
Pharmacist: Dispense the Patient information sheet

Donepezil HydrochlorideDonepezil Hydrochloride TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Donepezil HydrochlorideDonepezil Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!