DOMPERIDONE
CBSCHEM LIMITED
DOMPERIDONE
FULL PRESCRIBING INFORMATION
domperidone60.jpg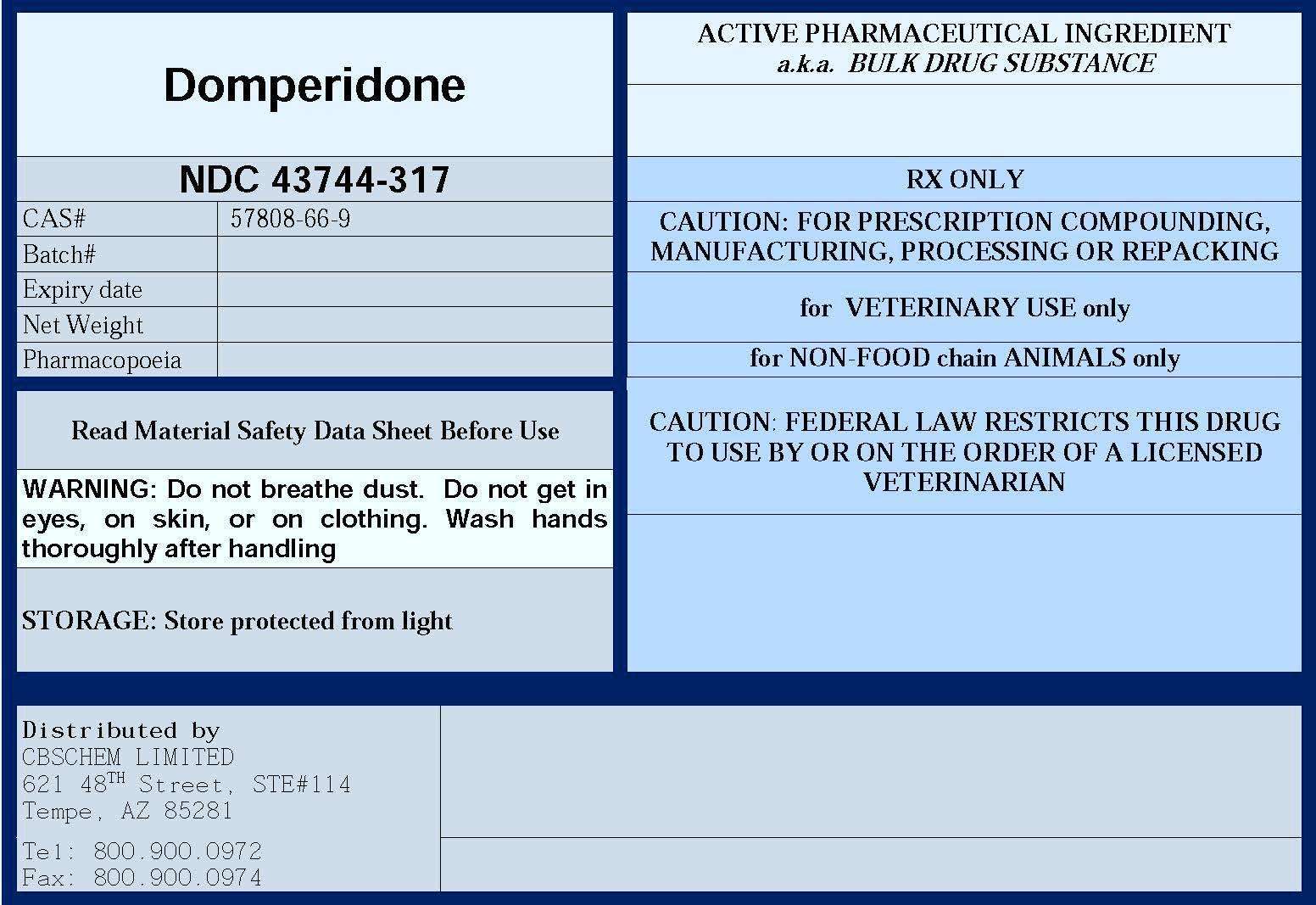
DOMPERIDONE
DOMPERIDONE POWDER
Product Information
|
|
Product Type
|
Bulk ingredient |
Item Code (Source)
|
NDC:43744-317 |
|
Route of Administration
|
NOT APPLICABLE |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
Domperidone DOMPERIDONE |
|
1 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:43744-317-10 |
1 in 1 DRUM |
|
|
|
2 |
NDC:43744-317-11 |
5 in 1 DRUM |
|
|
|
3 |
NDC:43744-317-15 |
10 in 1 DRUM |
|
|
|
4 |
NDC:43744-317-20 |
15 in 1 DRUM |
|
|
|
5 |
NDC:43744-317-22 |
20 in 1 DRUM |
|
|
|
6 |
NDC:43744-317-25 |
25 in 1 DRUM |
|
|
|
7 |
NDC:43744-317-30 |
50 in 1 DRUM |
|
|
|
8 |
NDC:43744-317-33 |
75 in 1 DRUM |
|
|
|
9 |
NDC:43744-317-35 |
100 in 1 DRUM |
|
|
|
10 |
NDC:43744-317-50 |
250 in 1 DRUM |
|
|
|
11 |
NDC:43744-317-55 |
500 in 1 DRUM |
|
|
|
12 |
NDC:43744-317-61 |
1000 in 1 DRUM |
|
|
|
13 |
NDC:43744-317-62 |
2000 in 1 DRUM |
|
|
|
14 |
NDC:43744-317-65 |
5000 in 1 DRUM |
|
|
|
15 |
NDC:43744-317-70 |
10000 in 1 DRUM |
|
|
|
16 |
NDC:43744-317-80 |
20000 in 1 DRUM |
|
|
|
17 |
NDC:43744-317-85 |
25000 in 1 DRUM |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2013-06-24 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!

