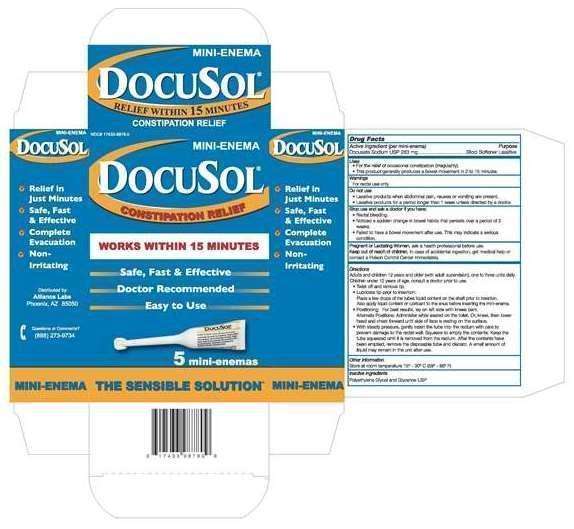
Docusol
Enemeez Inc. DBA Summit Pharmaceuticals
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Docusol Uses
- Warnings
- Ask a doctor before use if:
- Stop use and ask a doctor if you:
- Pregnant or Lactating Women
- Keep out of reach of children
- Docusol Uses
- Directions
- Other Information
- Inactive Ingredients
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient (per mini enema)
Docusate Sodium USP 283 mg.............................Stool Softener Laxative
Docusol Uses
- For relief of occasional constipation (irregularity).
- This product generally produces a bowel movement in 2 to 15 minutes.
Warnings
For rectal use only.
Ask a doctor before use if:
- abdominal pain, nausea or vomiting are present.
- Laxative products are needed for a period longer than 1 week unless directed by a doctor.
Stop use and ask a doctor if you:
- Rectal bleeding.
- Noticed a sudden change in bowel habits that persist over a period of 2 weeks.
- Failed to have a bowel movement after use. This may indicate a serious condition.
Pregnant or Lactating Women
Ask a health professional before use.
Keep out of reach of children
In case of accidental ingestion or overdose, get medical help or contact a Poison Control Center immediately.
Docusol Uses
- For relief of occasional constipation (irregularity).
- This product generally produces a bowel movement in 2 to 15 minutes.
Directions
Adults and children 12 years of age and older (with adult supervision) one to three units daily. Children under 12 years of age, consult a doctor prior to use.
- Twist off and remove tip.
- Lubricate tip prior to insertion: Place a few drops of the tubes liquid content on the shaft prior to insertion. Also apply tubes liquid or lubricant to the anus before inserting the mini-enema.
- Positioning: For best results, lay on the left side with knees bent. Alternate Positions:Administer while seated on the toilet. Or, k neel, then lower head and chest forward until side of face is resting on the surface.
- With steady pressure, gently insert the tube into the rectum with care to prevent damage to the rectal wall. Squeeze to empty the contents. Keep the tube squeezed until it is removed from the rectum. After the contents have been emptied, remove the disposable tube and discard. A small amount of liquid may remain in the unit after use.
Other Information
Other Information Store at room temperature 15°-30° C (59°-86° F)
Inactive Ingredients
Polyethylene Glycol and Glycerin USP
Questions or Comments: 1-888-273-9734
Distributed by:
Alliance Labs, L.L.C
Phoenix, AZ 85050
Docusoldocusate sodium LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||