DOCUSATE SODIUM
DOCUSATE SODIUM
FULL PRESCRIBING INFORMATION: CONTENTS*
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- INDICATIONS & USAGE SECTION
- WARNINGS SECTION
- DOSAGE & ADMINISTRATION SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- OTC - KEEP OUT OF REACH OF CHILDREN SECTION
- INACTIVE INGREDIENT SECTION
FULL PRESCRIBING INFORMATION
OTC - ACTIVE INGREDIENT SECTION
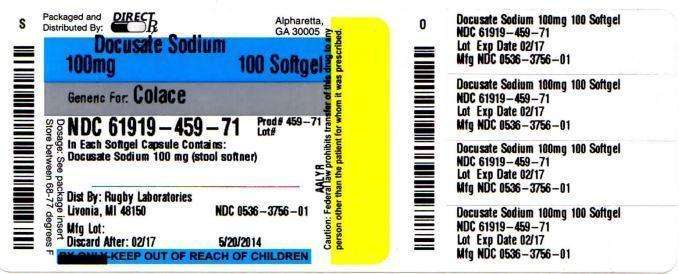
Docusate Sodium 100 mg
OTC - PURPOSE SECTION
Stool softener
INDICATIONS & USAGE SECTION
- for prevention of dry, hard stools
- for relief of occasional constipation
This product generally produces a bowel movement within 12 to 72 hours.
WARNINGS SECTION
Do not use
- if you are currently taking mineral oil, unless directed by a doctor
- when abdominal pain, nausea, or vomiting are present
- for longer than 1 week, unless directed by a doctor
Ask a doctor before use if
if you notice a sudden change in bowel habits that persists over a period of 2 weeks.
Stop use and ask a doctor if
- you have rectal bleeding
- you fail to have a bowel movement after use
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health care professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
| adults and children over 12 years of age | take 1 to 3 softgels preferably at bedtime |
| children 6-12 years of age | take 1 softgel at bedtime |
| children under 6 years | ask a doctor |
Other information
- each softgel contains: sodium 6 mg
- store between 15º-30ºC (59º-86ºF)
Questions or comments?
1-800-645-2158
Principal Display Panel
COMPARE TO ACTIVE INGREDIENT IN COLACE®*
NON-HABIT FORMING
Stool Softener Laxative
Docusate Sodium USP, 100 mg
SOFTGEL CAPSULES
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*Rugby Laboratories is not affiliated with the owner of the trademark Colace®.
Distributed by: Rugby Laboratories
31778 Enterprise Drive
Livonia, MI 48150
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
In case of overdose, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENT SECTION
edible white ink, FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol special.
DOCUSATE SODIUMDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||