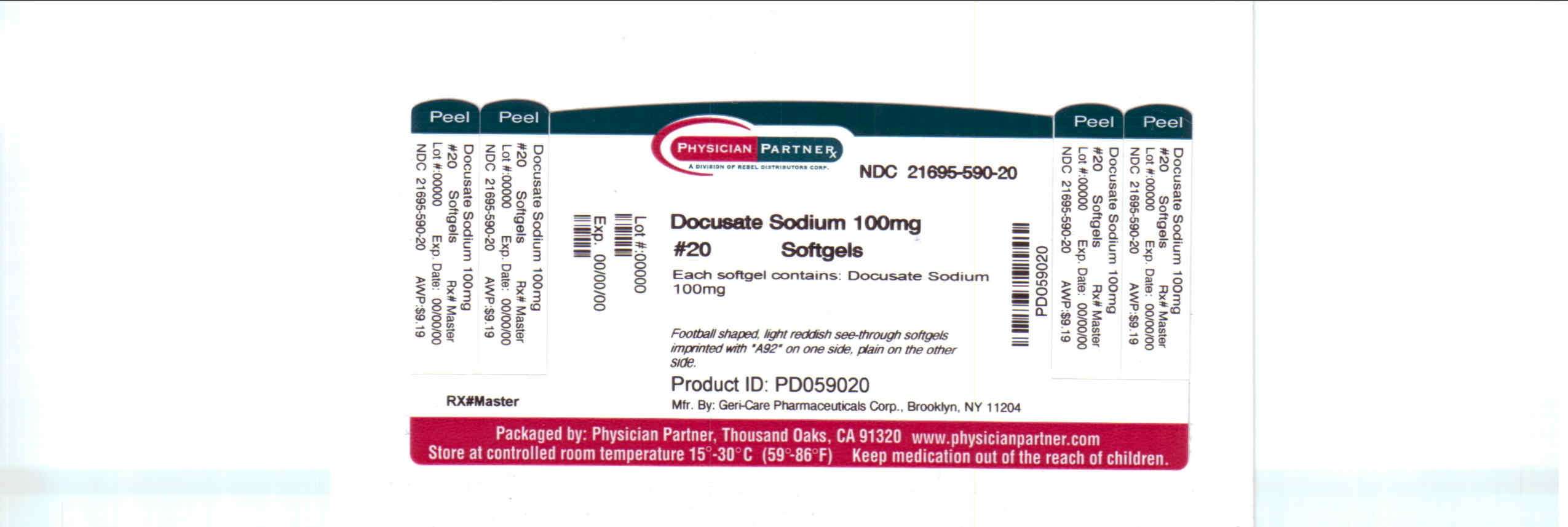
Docusate Sodium
Docusate Sodium Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Docusate Sodium Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Docusate Sodium 100mg
Purpose
Stool Softener
Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control center right way.
Docusate Sodium Uses
Prevents / relieves dry hard stool. Results usually occur 1 to 3 days after the first dose.
Warnings
Do not use for more than one week unless directed by a doctor.
Ask a doctor before use if you have abdominal pain, nausea, or vomiting; have noticed a sudden change in bowel habits that lasts over 2 weeks,; are taking mineral oil.
Stop use and ask a doctor if you have no bowel movement within 3 days; you have rectal bleeding. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Tamper Evident: Do not use if imprinted seal under cap is missing or broken.
Directions
Adults and children 12 years and older: take 1-2 softgels daily until first bowel movement; 1 softgel daily thereafter, or as directed by a doctor.
Children under 12: consult a doctor.
Do not exceed recommended dose.
Other Information
Each softgel contains sodium 5mg.
Product from Canada or USA
Store at room temperature, 15°C-30°C (59°F-86°F)
Inactive ingredients
may contain cirtic acid, D&C red no. 33, D&C yellow no. 10, ethyl vanillin, FD&C blue no. 1, FD&C re no. 40 FD&C yellow no. 6, gelatin, glycerin, edible ink, mannitol, methylparaben, polyethylene glycol, propylene glycol, propylparaben, sorbitol, water.
Distributed by:
Geri-Care Pharmaceuticals Corp.
Brooklyn, NY 11204
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
Package/Label Principal Display Panel
Docusate SodiumDocusate Sodium CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||