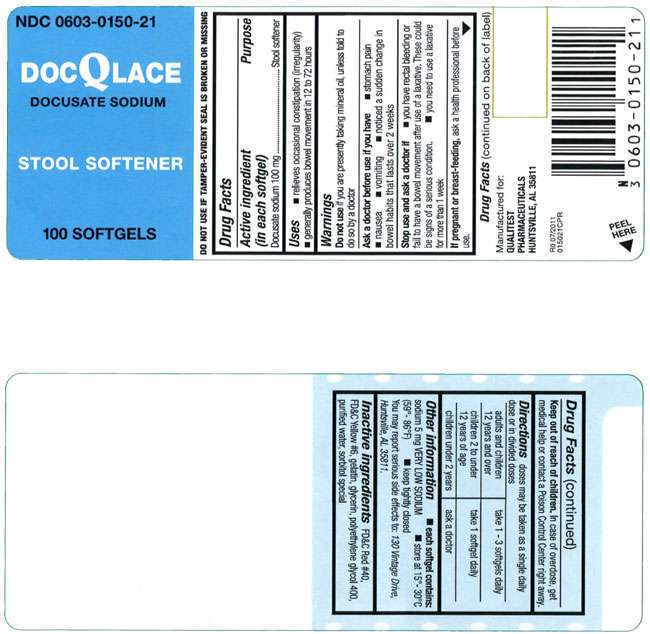
DocQLace
DocQLace
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Docusate sodium 100 mg
Stool softener
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Do not use if you are presently taking mineral oil, unless told to do so by a doctor
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding, ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
doses may be taken as a single daily dose or in divided doses
| adults and children 12 years and over | take 1-3 softgels daily |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
- each softgel contains: sodium 5 mg VERY LOW SODIUM
- store at 15°-30°C (59°-86°F)
- keep tightly closed
You may report serious side effects to: 130 Vintage Drive, Huntsville, AL 35811.
FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol 400, purified water, sorbital special
Manufactured for:
QUALITEST PHARMACEUTICALS
HUNTSVILLE, AL 35811
R0 07/2011
015021CPR
PRINCIPAL DISPLAY PANEL
DocQLacedocusate sodium CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!