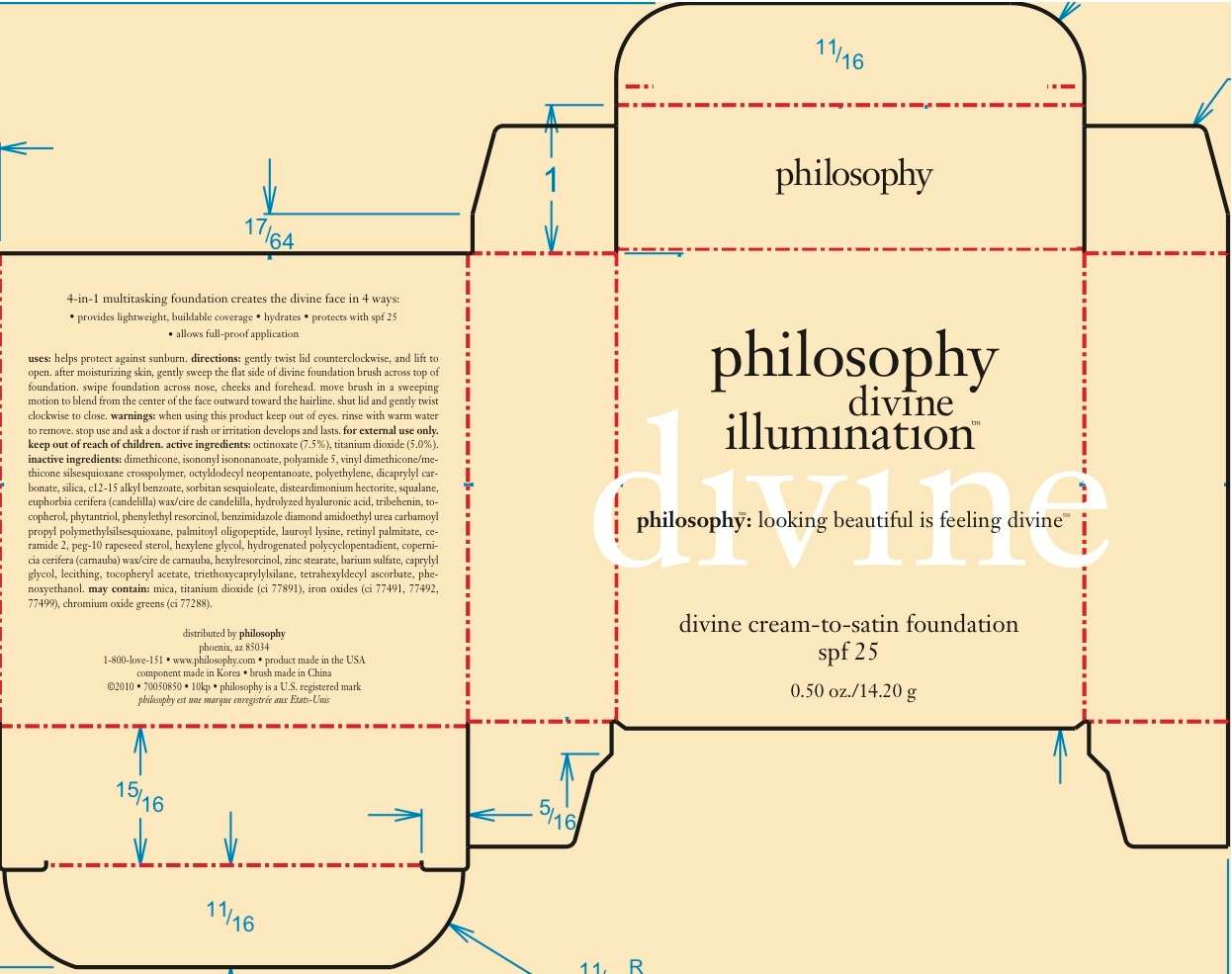
Divine Lift Foundation SPF
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
OCTINOXATE (7.5%)
TITANIUM DIOXIDE (5.0%)
Purpose
HELPS TO PROTECT AGAINST SUNBURN.
WHEN USING THIS PRODUCT KEEP OUT OF EYES. RINSE WITH WARM WATER TO REMOVE. FOR EXTERNAL USE ONLY.
STOP USE AND ASK A DOCTOR IF RASH OR IRRITATION DEVELOPS AND LASTS.
KEEP OUT OF REACH OF CHILDREN
GENTLY TWIST LID COUNTERCLOCKWISE, AND LIFT TO OPEN. AFTER MOISTURIZING SKIN, GENTLY SWEEP THE FLAT SIDE OF DIVINE FOUNDATION BRUSH ACROSS TOP OF FOUNDATION, SWIPE FOUNDATION ACROSS NOSE, CHEEKS AND FOREHEAD. MOVE BRUSH IN A SWEEPING MOTION TO BLEND FROM THE CENTER OF THE FACE OUTWARD TOWARDS THE HAIRLINE. SHUT LID AND GENTLY TURN CLOCKWISE TO CLOSE.