Divalproex Sodium Delayed Release
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- DIVALPROEX SODIUM DELAYED RELEASE DESCRIPTION
- PHARMACODYNAMICS
- PHARMACOKINETICS
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DIVALPROEX SODIUM DELAYED RELEASE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DIVALPROEX SODIUM DELAYED RELEASE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DIVALPROEX SODIUM DELAYED RELEASE DESCRIPTION
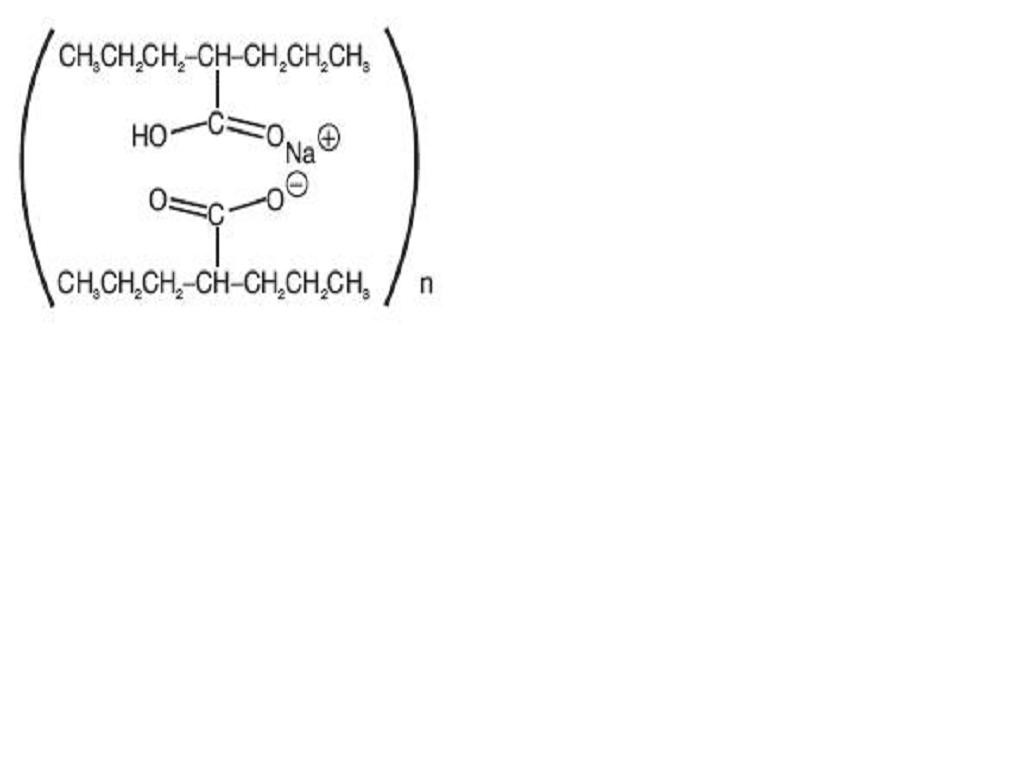
PHARMACODYNAMICS
PHARMACOKINETICS
Absorption/BioavailabilityDOSAGE AND ADMINISTRATION
Distribution
Protein Binding
PRECAUTIONSDrug Interactions
CNS Distribution
Metabolism
Elimination
Special Populations
Effect of Age
Neonates
Children
Elderly
DOSAGE AND ADMINISTRATION
Effect of Gender
Effect of Race
Effect of Disease
Liver Disease
BOXED WARNINGCONTRAINDICATIONSWARNINGS
Renal Disease
Plasma Levels and Clinical Effect
Epilepsy
Mania
DOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY
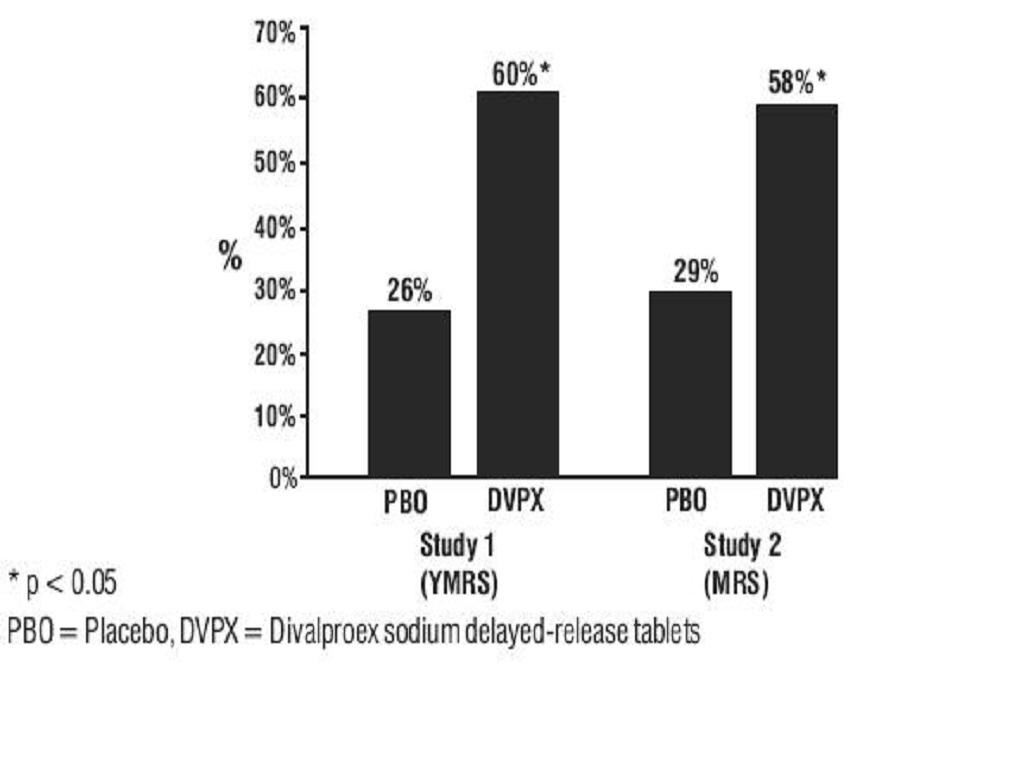
Mania*YMRS Total ScoreGroupBaseline*BL to Wk 3DifferenceBPRS-A Total ScoreGroupBaseline*BL to Wk 3DifferenceGAS ScoreGroupBaseline*BL to Wk 3Difference
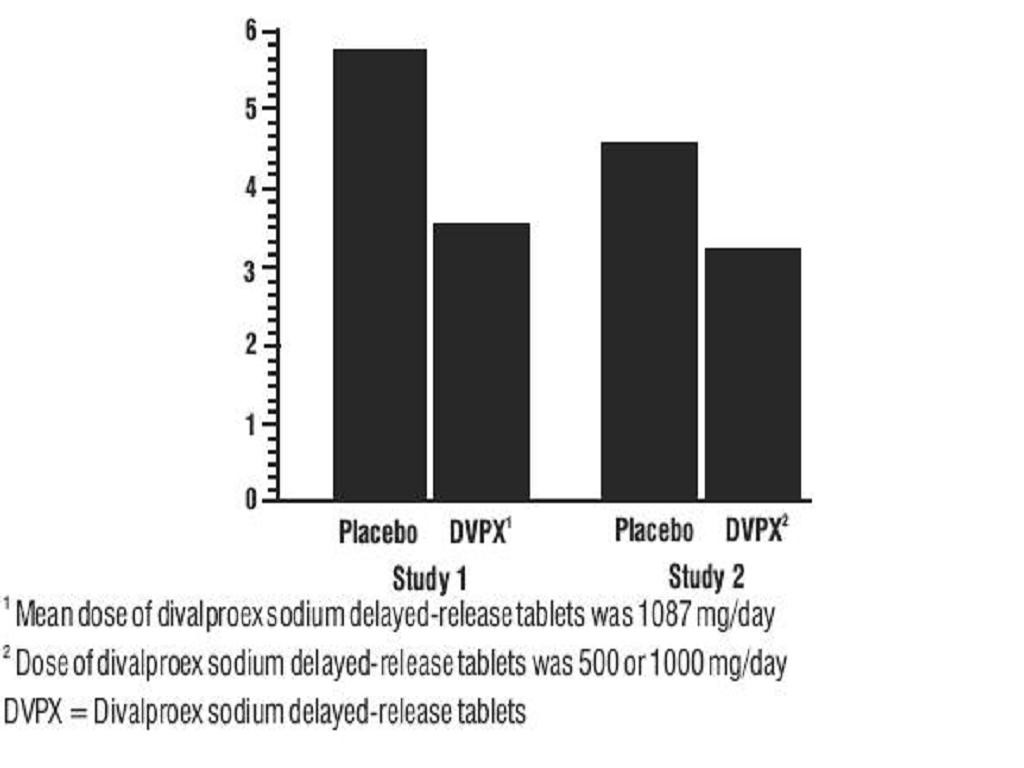
*MRS Total ScoreGroupBaseline*BL to Day 21DifferenceMSS Total ScoreGroupBaseline*BL to Day 21DifferenceBIS Total ScoreGroupBaseline*BL to Day 21Difference
Migraine
Epilepsy
*Add-on TreatmentNumber of PatientsBaseline IncidenceExperimental Incidence*
*TreatmentNumber ofBaseline IncidenceRandomized PhasePatientsIncidence*
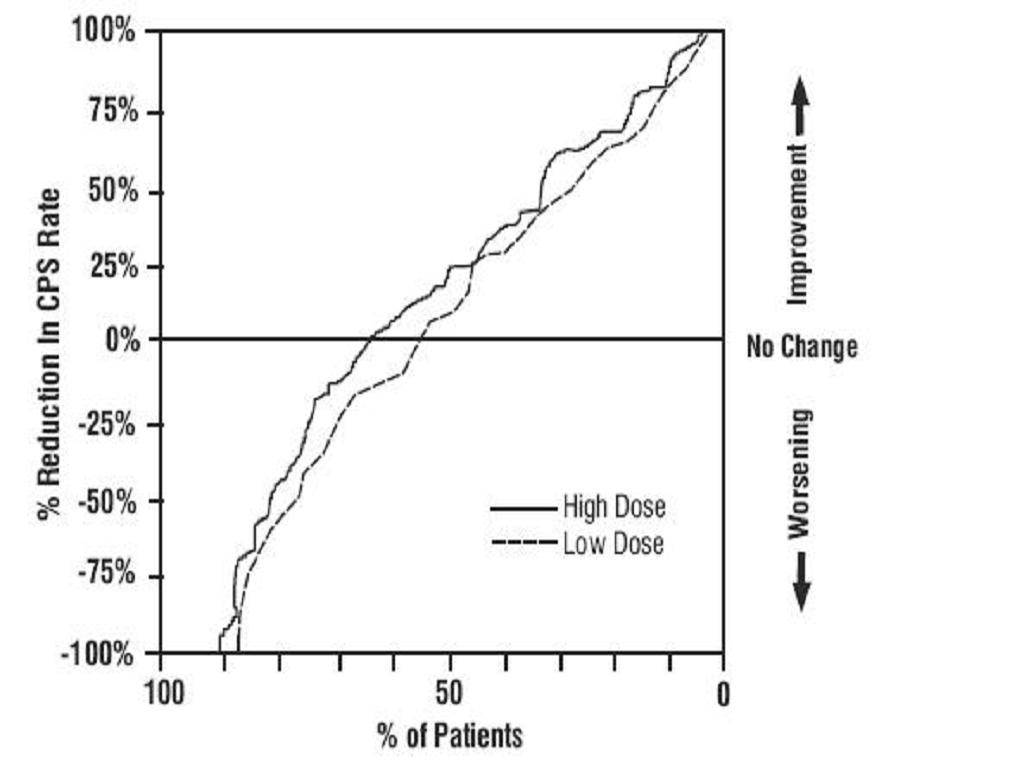
INDICATIONS & USAGE
ManiaClinical TrialsCLINICAL PHARMACOLOGY
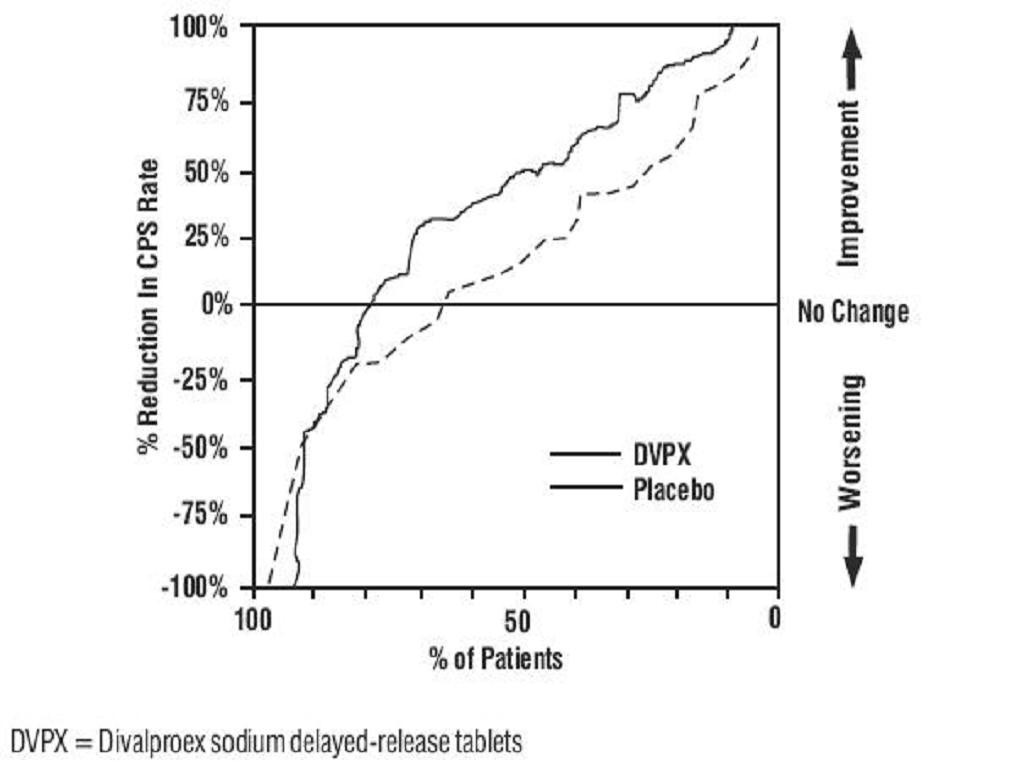
Epilepsy
Migraine
WARNINGSUsage In PregnancyPRECAUTIONSInformation for Patients
WARNINGS
DIVALPROEX SODIUM DELAYED RELEASE CONTRAINDICATIONS
WARNINGS
WARNINGS
HepatotoxicityPancreatitis
BOXED WARNING
Urea Cycle Disorders (UCD)
CONTRAINDICATIONSPRECAUTIONS
Usage In Pregnancy
Human Data
Congenital Malformations
Neural Tube Defects
Other Adverse Pregnancy Effects
PRECAUTIONSGENERALWARNINGS
WARNINGSHEPATOTOXICITYBOX WARNING
Animal Data
Suicidal Behavior and Ideation
Indication PlaceboDrug PatientsRelative Risk: Incidence ofRisk Difference:Patients w/with EventsEvents in Drug Patients/Additional Drug PatientsEvents PerPer 1000Incidence in Placebo Patientswith Events Per 10001000 PatientsPatientsPatients
Interaction with Carbapenem Antibiotics
Drug Interactions
Somnolence in the Elderly
DOSAGE AND ADMINISTRATION
Thrombocytopenia
PRECAUTIONS
PRECAUTIONS
Hepatic DysfunctionBOXED WARNINGCONTRAINDICATIONSWARNINGS
Pancreatitis
BOXED WARNINGWARNINGS
Hypothermia
Drug InteractionsTopiramate
Hyperammonemia
CONTRAINDICATIONSWARNINGSUrea Cycle Disorders (UCD)PRECAUTIONSHyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use
CONTRAINDICATIONSWARNINGSUrea Cycle DisordersPRECAUTIONSHyperammonemia
Hyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use
PRECAUTIONSHypothermiaCONTRAINDICATIONSWARNINGSUrea Cycle DisordersPRECAUTIONSHyperammonemia
General
WARNINGS
PRECAUTIONSDrug Interactions
Multi-organ Hypersensitivity Reaction
INFORMATION FOR PATIENTS
PancreatitisHyperammonemia
PRECAUTIONSHyperammonemia
CNS Depression
Birth Defects
PRECAUTIONSPregnancy
Suicidal Thinking and Behavior
WARNINGS
Multi-organ Hypersensitivity Reaction
PRECAUTIONSMulti-organ Hypersensitivity Reaction
DRUG INTERACTIONS
Effects of Co-Administered Drugs on Valproate ClearanceDrugs for Which a Potentially Important Interaction Has Been Observed
Aspirin
Felbamate
Carbapenem Antibiotics
WARNINGS
Rifampin
Drugs for Which Either No Interaction or a Likely Clinically Unimportant Interaction Has Been Observed
Antacids
Chlorpromazine
Haloperidol
Cimetidine and Ranitidine
Effects of Valproate on Other Drugs
Drugs for Which a Potentially Important Valproate Interaction Has Been Observed
Amitriptyline/Nortriptyline
Carbamazepine/carbamazepine 10,11-Epoxide
Clonazepam
Diazepam
Ethosuximide
Lamotrigine
Phenobarbital
Phenytoin
Tolbutamide
Topiramate
CONTRAINDICATIONSWARNINGSUrea Cycle DisordersPRECAUTIONSHyperammonemia and Encephalopathy Associated with Concomitant Topiramate UsePRECAUTIONSHypothermiaHyperammonemia
Warfarin
Zidovudine
Drugs for Which Either No Interaction or a Likely Clinically Unimportant Interaction Has Been Observed
Acetaminophen
Clozapine
Lithium
Lorazepam
Oral Contraceptive Steroids
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Fertility
PREGNANCY
Pregnancy category DNURSING MOTHERS
PEDIATRIC USE
BOXED WARNINGGERIATRIC USE
WARNINGSSomnolence in the ElderlyDOSAGE AND ADMINISTRATION
DIVALPROEX SODIUM DELAYED RELEASE ADVERSE REACTIONS
Mania*
*Adverse EventDivalproex Sodium Delayed-Placebo (n = 97)Release Tablets (n = 89)
Migraine
*
*Body System EventDivalproex Sodium Delayed-Placebo (N = 81)Gastrointestinal SystemRelease Tablets (N= 202)Nervous SystemOther
Epilepsy
Body System/EventDivalproex Sodium Delayed-ReleasePlacebo (%)Tablets (%) (n = 77)(n = 70)Body as a WholeGastrointestinal SystemNervous SystemRespiratory SystemOther
*
*Body System/EventHigh Dose (%) (n = 131)Low Dose (%) (n = 134)Body as a WholeDigestive SystemHemic/Lymphatic SystemMetabolic/NutritionalNervous SystemRespiratory SystemSkin and AppendagesSpecial Senses
Other Patient Populations
Gastrointestinal
CNS Effects
WARNINGSUrea Cycle DisordersPRECAUTIONS
Dermatologic
PRECAUTIONSDrug Interactions
Psychiatric
Musculoskeletal
Hematologic
PRECAUTIONSGeneralDrug Interactions
Hepatic
WARNINGS
Endocrine
PRECAUTIONS
Pancreatic
WARNINGS
Metabolic
PRECAUTIONS
Genitourinary
Special Senses
Other
OVERDOSAGE
DOSAGE & ADMINISTRATION
ManiaEpilepsy
PRECAUTIONSDrug Interactions
Complex Partial Seizures
Monotherapy (Initial Therapy)
Conversion to Monotherapy
Adjunctive Therapy
CLINICAL STUDIESDrug InteractionsPRECAUTIONSDrug Interactions
Simple and Complex Absence Seizures
CLINICAL PHARMACOLOGY
PRECAUTIONS
Migraine
General Dosing Advice
Dosing in Elderly Patients
WARNINGS
Dose-Related Adverse Events
PRECAUTIONS
G.I. Irritation
HOW SUPPLIED
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATION-
● Women taking any of these medications who are planning to get pregnant should discuss the treatment options with their doctor.
-
● Information For Women Who Become Pregnant
-
● Other Important Information
-
● If you have taken more than the prescribed dose of your medication, contact your hospital emergency room or local poison center immediately.
-
● Your medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
-
● Facts About Birth Defects
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION



Divalproex Sodium Delayed ReleaseDivalproex Sodium Delayed Release TABLET, DELAYED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!