Divalproex Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
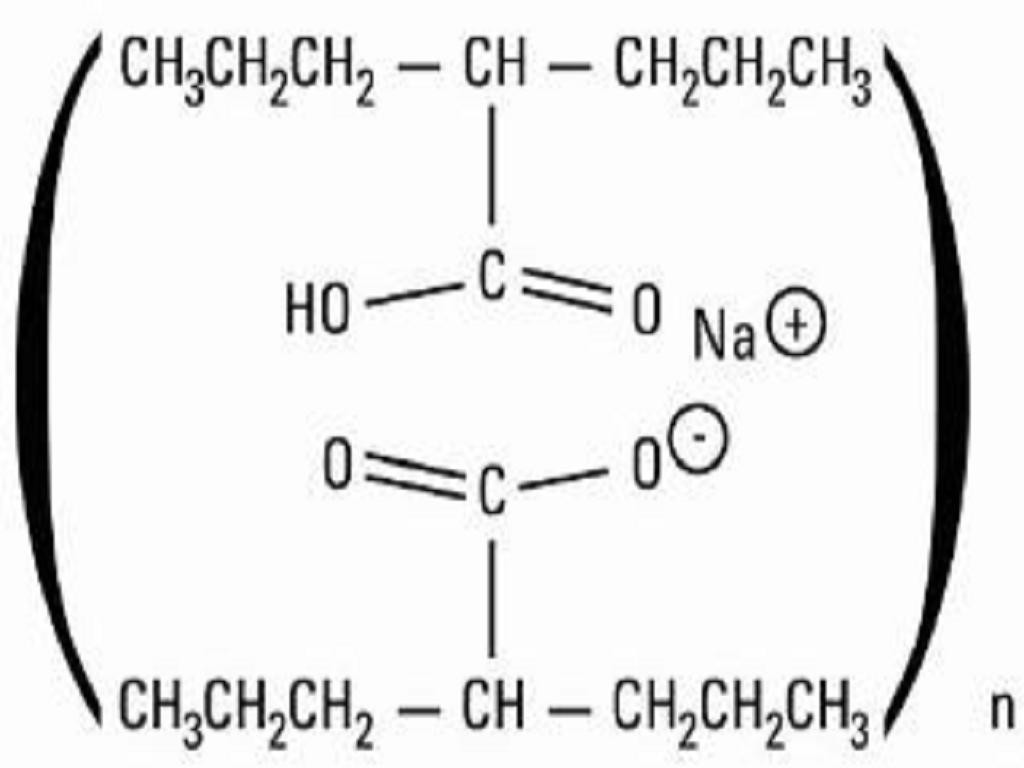
- DIVALPROEX SODIUM DESCRIPTION
- INACTIVE INGREDIENT
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- DIVALPROEX SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- DIVALPROEX SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DIVALPROEX SODIUM DESCRIPTION
INACTIVE INGREDIENT
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Absorption/BioavailabilityDOSAGE AND ADMINISTRATION
Distribution
Protein Binding
Drug Interactions
CNS Distribution
Metabolism
Elimination
Special Populations
Effect of Age
DOSAGE AND ADMINISTRATION
Effect of Gender
Effect of Race
Effect of Disease
BOXED WARNINGCONTRAINDICATIONSWARNINGS
Plasma Levels and Clinical Effect
Epilepsy
Mania
DOSAGE AND ADMINISTRATION
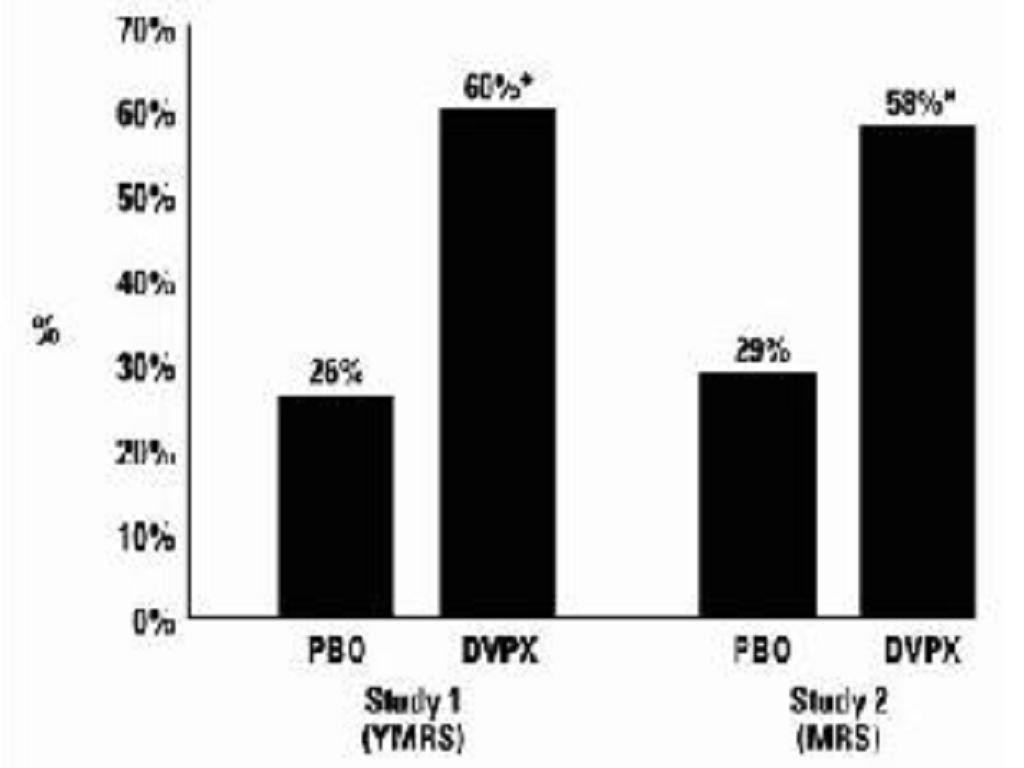
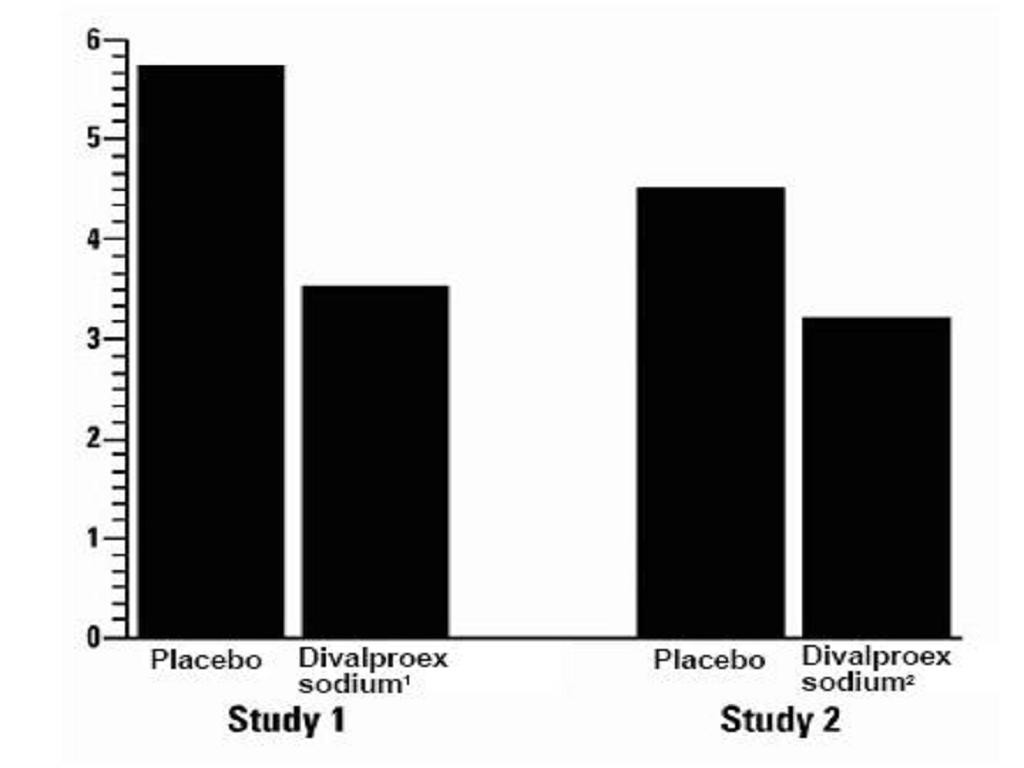
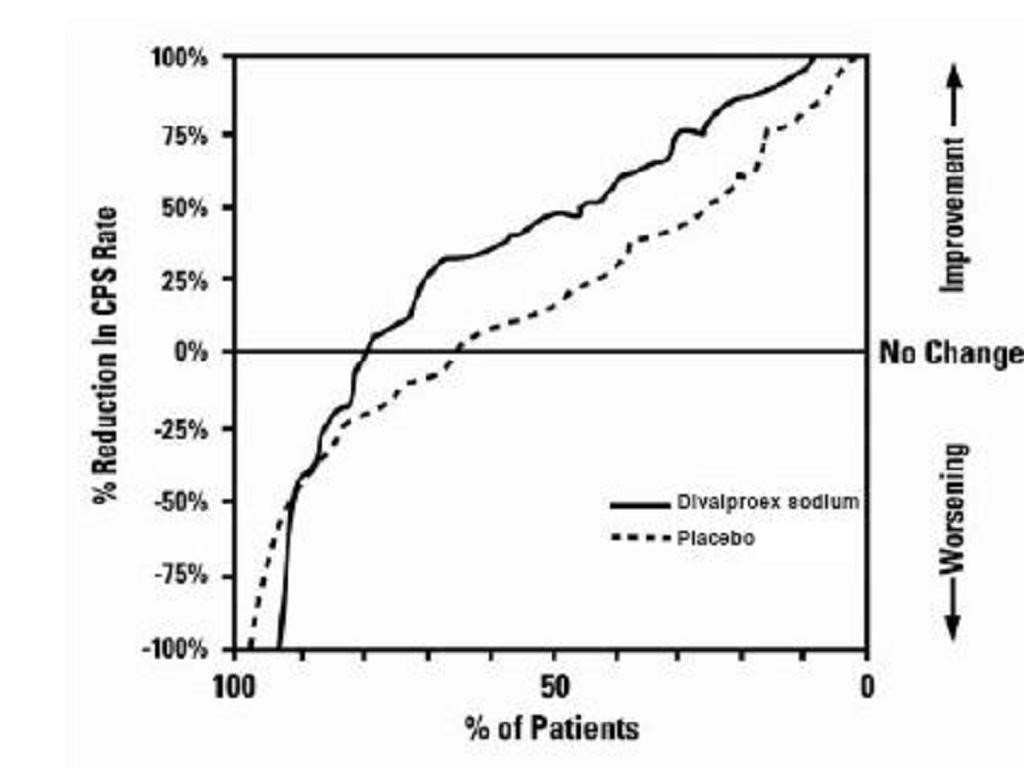
Clinical Trials
Mania
****
*******
Migraine
Epilepsy
**
**

INDICATIONS & USAGE
ManiaClinical Trials
Epilepsy
Migraine
Usage In PregnancyInformation for Patients
WARNINGS
DIVALPROEX SODIUM CONTRAINDICATIONS
WARNINGS
WARNINGS
HepatotoxicityHepatic failure resulting in fatalities has occurred in patients receiving valproic acid. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Liver function tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months. However, healthcare providers should not rely totally on serum biochemistry since these tests may not be abnormal in all instances, but should also consider the results of careful interim medical history and physical examination.
Caution should be observed when administering divalproex sodium products to patients with a prior history of hepatic disease. Patients on multiple anticonvulsants, children, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease may be at particular risk. Experience has indicated that children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those with the aforementioned conditions. When divalproex sodium is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. Above this age group, experience in epilepsy has indicated that the incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
The drug should be discontinued immediately in the presence of significant hepatic dysfunction, suspected or apparent. In some cases, hepatic dysfunction has progressed in spite of discontinuation of drug.
Pancreatitis
BOXED WARNING
Urea Cycle Disorders (UCD)
CONTRAINDICATIONSPRECAUTIONS
Usage In Pregnancy
HUMAN DATA
Congenital Malformations
Neural Tube Defects
GENERALWARNINGS
HEPATOTOXICITYBOX WARNING
ANIMAL DATA
Suicidal Behavior and Ideation
Interaction with Carbapenem Antibiotics
Drug Interations
Somnolence in the Elderly
DOSAGE AND ADMINISTRATION
Thrombocytopenia
PRECAUTIONS
PRECAUTIONS
Hepatic DysfunctionBOXED WARNINGCONTRAINDICATIONSWARNINGS
Pancreatitis
BOXED WARNINGWARNINGS
Hypothermia
Drug Interactions
Hyperammonemia
HypothermiaCONTRAINDICATIONSUrea Cycle Disorders(UCD)Hyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use)
CONTRAINDICATIONSUrea Cycle DisordersHyperammonemia
Hyperammonemia and Encephalopathy Associated with Concomitant Topiramate Use
HypothermiaCONTRAINDICATIONSUrea Cycle DisordersHyperammonemia
General
WARNINGS
Drug Interactions
Multi-organ Hypersensitivity Reaction
INFORMATION FOR PATIENTS
PancreatitisHyperammonemia
Hyperammonemia
CNS Depression
Birth Defects
Pregnancy
Suicidal Thinking and Behavior
WARNINGS
Multi-organ Hypersensitivity Reaction
Multi-organ Hypersensitivity Reaction
DRUG INTERACTIONS
WARNINGS
CONTRAINDICATIONSUrea CycleDisordersHyperammonemiaHyperammonemia and Encephalopathy Associated with Concomitant TopiramateUseHypothermiaHyperammonemia
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGSNURSING MOTHERS
PEDIATRIC USE
BOXED WARNINGGERIATRIC USE
Somnolence in the ElderlyDOSAGE AND ADMINISTRATION
DIVALPROEX SODIUM ADVERSE REACTIONS
Mania*
*
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Migraine
Table 3
*
*
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Epilepsy
*
*
Body as a Whole
Cardiovascular System
Digestive System
Hemic and Lymphatic System
Metabolic and Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Other Patient Populations
Gastrointestinal
CNS Effects
WARNINGSPRECAUTIONS
Dermatologic
PRECAUTIONS
Psychiatric
Musculoskeletal
Hematologic
GeneralDrug Interactions
Hepatic
WARNINGS
Endocrine
PRECAUTIONS
Pancreatic
WARNINGS
Metabolic
PRECAUTIONS
Genitourinary
Special Senses
Other
OVERDOSAGE
DOSAGE & ADMINISTRATION
ManiaEpilepsy
PRECAUTIONS
Complex Partial Seizures
Monotherapy (Initial Therapy)
Conversion to Monotherapy
Adjunctive Therapy
CLINICAL STUDIESDrug InteractionsPRECAUTIONS
Simple and Complex Absence Seizures
CLINICAL PHARMACOLOGY
PRECAUTIONS
Migraine
General Dosing Advice
Dosing in Elderly Patients
WARNINGS
Dose-Related Adverse Events
PRECAUTIONS
G.I. Irritation
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Patient Information LeafletImportant Information for Women Who Could Become Pregnant About the Use of Divalproex Sodium Delayed-Release Tablets.
Information For Women Who Could Become Pregnant
Before using divalproex sodium delayed-release tablets, women who can become pregnant should consider the fact that divalproex sodium delayed-release tablets have been associated with birth defects, in particular, with spina bifida and other defects related to failure of the spinal canal to close normally. Approximately 1 to 2% of children born to women with epilepsy taking divalproex sodium in the first 12 weeks of pregnancy had these defects (based on data from the Centers for Disease Control, a U.S. agency based in Atlanta). The incidence in the general population is 0.1 to 0.2%.
Divalproex sodium delayed-release tablets have also been associated with other birth defects such as defects of the heart, the bones, and other parts of the body. Information suggests that birth defects may be more likely to occur with divalproex sodium delayed-release tablets than some other drugs that treat your medical condition.
Information For Women Who Are Planning to Get Pregnant
-
● Women taking divalproex sodium delayed-release tablets who are planning to get pregnant should discuss the treatment options with their doctor.
-
● If you become pregnant while taking divalproex sodium delayed-release tablets you should contact your doctor immediately.
-
● Your medication should be taken exactly as prescribed by your doctor to get the most benefit from your medication and reduce the risk of side effects.
-
● If you have taken more than the prescribed dose of your medication, contact your hospital emergency room or local poison center immediately.
-
● Your medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Divalproex SodiumDivalproex Sodium TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!