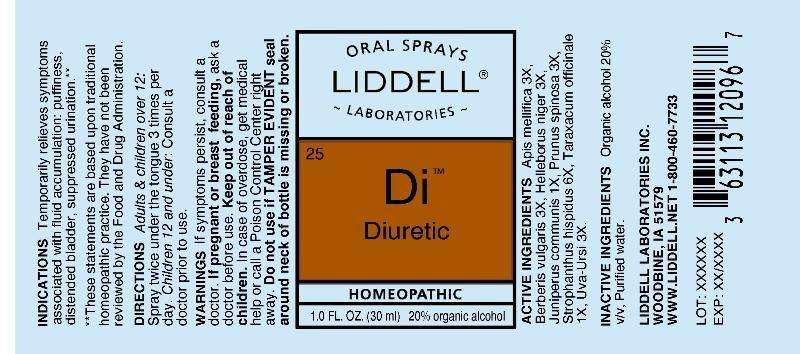
Diuretic
Liddell Laboratories, Inc.
Apotheca Company
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS:
- INDICATIONS:
- WARNINGS:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS:
Apis mellifica 3X, Berberis vulgaris 3X, Helleborus niger 3X, Juniperus communis 1X, Prunus spinosa 3X, Strophanthus hispidus 6X, Taraxacum officinale 1X, Uva-ursi 3X.
INDICATIONS:
Temporarily relieves symptoms associated with fluid accumulation: pussiness, distended bladdder, suppressed urination.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
WARNINGS:
If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
DIRECTIONS:
Adults and children over 12: Spray twice under the tongue 3 times per day. Children 12 and under: Consult a doctor prior to use.
INACTIVE INGREDIENTS:
Organic alcohol 20% v/v, Purrified water.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or call a Poison Control Center right away.
INDICATIONS:
Temporarily relieves symptoms associated with fluid accumulation: pussiness, distended bladdder, suppressed urination.**
**These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
QUESTIONS:
LIDDELL LABORATORIES INC.
WOODBINE, IA 51579
WWW.LIDDELL.NET
1-800-460-7733
PACKAGE LABEL DISPLAY
ORAL SPRAYS
LIDDELL LABORATORIES
25
Di
Diuretic
HOMEOPATHIC
1.0 FL. OZ. (30 ml)
DiureticApis Melifica, Berberis vulgaris, Nelleborus niger, Juniperus communis, Prunus spinosa, Strophanthus hispidus, Taraxacum offidcinale, Uva-Ursi, SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||