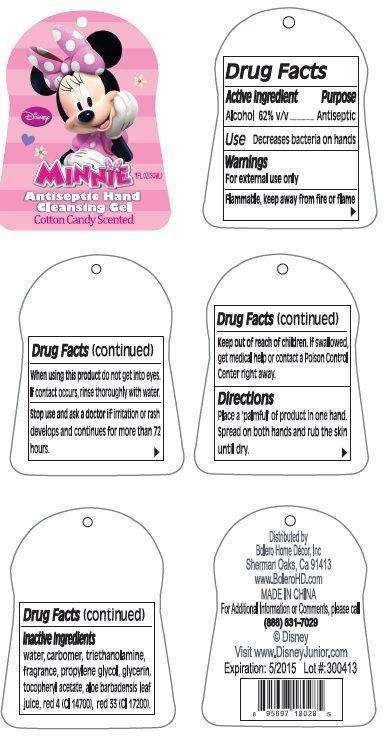
Disney Minnie Antiseptic Hand Cleansing Cotton Candy Scented
Disney Minnie Antiseptic Hand Cleansing Gel Cotton Candy Scented
FULL PRESCRIBING INFORMATION: CONTENTS*
- Disney Minnie Antiseptic Hand Cleansing Gel Cotton Candy Scented
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive Ingredients
- Disney Minnie Antiseptic Hand Cleansing Gel
- Disney Minnie Antiseptic Hand Cleansing Gel Cotton Candy Scented 1oz/30ml (57469-011-00)
FULL PRESCRIBING INFORMATION
Disney Minnie Antiseptic Hand Cleansing Gel Cotton Candy Scented
Active Ingredient
Alcohol 62% v/v
Purpose
Antiseptic
Use
Decreases bacteria on hands
Warnings
For external use only
Flammable, keep away from fire or flame
When using this product
do not get into eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask a doctor
if irritation or rash develops and continues for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Place a 'palmful' of product in one hand. Spread on both hands and rub the skin until dry.
Inactive Ingredients
water, carbomer, triethanolamine, fragrance, propylene glycol, glycerin, tocopheryl acetate, aloe barbadensis leaf juice, fragrance, red 4 (CI 14700), red 33 (CI 17200).
Disney Minnie Antiseptic Hand Cleansing Gel
Distributed by:
Bolero Home Decor, Inc.
Sherman Oaks, Ca 91413
www.BoleroHD.com
MADE IN CHINA
For additional Information
or Comments, please call
(888) 831-7029
© Disney
Visit www.DisneyJunior.com
Disney Minnie Antiseptic Hand Cleansing Gel Cotton Candy Scented 1oz/30ml (57469-011-00)
Disney Minnie Antiseptic Hand Cleansing Cotton Candy ScentedALCOHOL GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||