Diphenhydramine
Diphenhydramine Capsules
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each capsule)
- Purpose
- Diphenhydramine Uses
- Warnings
- Directions
- Diphenhydramine Other information
- Inactive ingredients
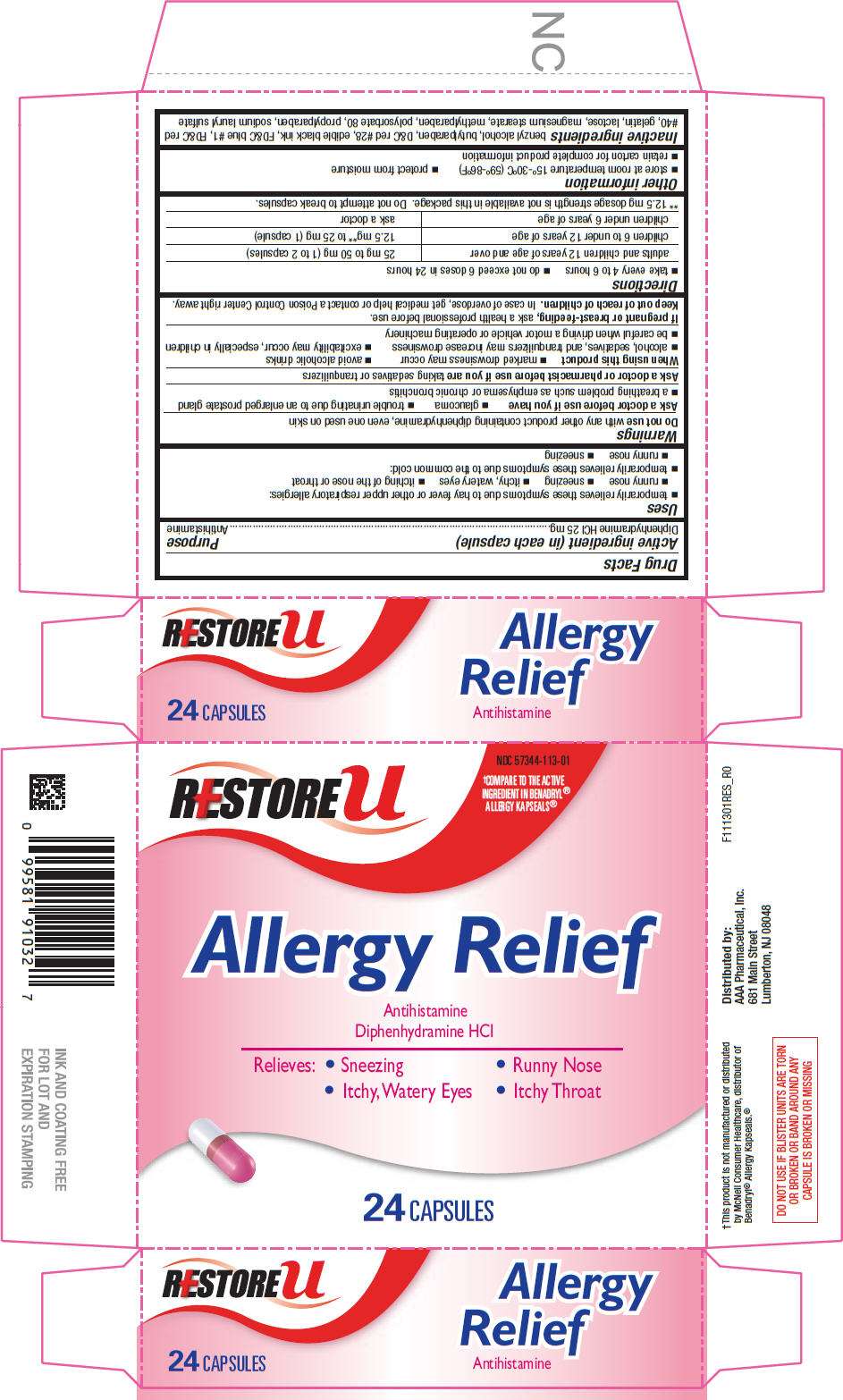
- PRINCIPAL DISPLAY PANEL - 24 Capsule Blister Pack Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (in each capsule)
Diphenhydramine HCl 25 mg
Purpose
Antihistamine
Diphenhydramine Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- excitability may occur, especially in children
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take every 4 to 6 hours
- do not exceed 6 doses in 24 hours
| adults and children 12 years of age and over | 25 mg to 50 mg (1 to 2 capsules) |
| children 6 to under 12 years of age | 12.5 mg |
| children under 6 years of age | ask a doctor |
Diphenhydramine Other information
- store at room temperature 15°-30°C (59°-86°F)
- protect from moisture
- retain carton for complete product information
Inactive ingredients
benzyl alcohol, butylparaben, D&C red #28, edible black ink, FD&C blue #1, FD&C red #40, gelatin, lactose, magnesium stearate, methylparaben, polysorbate 80, propylparaben, sodium lauryl sulfate
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
PRINCIPAL DISPLAY PANEL - 24 Capsule Blister Pack Carton
RESTORE u
NDC 57344-113-01
†COMPARE TO THE ACTIVE
INGREDIENT IN BENADRYL®
ALLERGY KAPSEALS®
Allergy Relief
Antihistamine
Diphenhydramine HCl
Relieves: • Sneezing • Runny Nose
• Itchy, Watery Eyes • Itchy Throat
24 CAPSULES
DiphenhydramineDIPHENHYDRAMINE HYDROCHLORIDE CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||