DiorSnow White Reveal Instant Spot Concealer SPF 50 Ivory 010
DiorSnow White Reveal Instant Spot Concealer SPF 50 Ivory 010
FULL PRESCRIBING INFORMATION
Active ingredient
WARNINGS: For external use only.
keep out of eyes
Discontinue use if skin irritation develops or increases. If irritation persists, consult a health care practitioner.
Keep out of reach of children.
DIORSNOW
WHITE REVEAL INSTANT SPOT CONCEALER
SPF 50 - PA+++
010
Dermatologist-tested on Asian skin.
F044341010
3348900973985
MADE IN FRANCE
Parfums Christian Dior
33, avenue Hoche
75008 Paris - France
In USA - 19E57 NY. NY 10022
www.dior.com
DIORSNOW
WHITE REVEAL INSTANT SPOT CONCEALER
010
SPF 50 - PA+++
Dior
Apply prior to sun exposure.
WARNINGS: For external use only. keep out of eyes. Discontinue use if skin irritation develops or increases. If irritation persists, consult a health care practitioner. Keep out of reach of children.
Contains: Oxybenzone
ACTIVE INGREDIENTS : OCTINOXATE (ETHYLHEXYL METHOXYCINNAMATE) 7.49 % - OXYBENZONE (BENZOPHENONE-3) 2.50 %
9M
MADE IN FRANCE
Parfums Christian Dior
75008 Paris - France
In USA - 19E57 NY. NY 10022
15 ml - NET WT. 0.63 OZ
010
CD DIORSNOW
WHITE REVEAL INSTANT SPOT CONCEALER
SPF 50 - PA+++
Dior
15 ml- NET WT.0.63 OZ

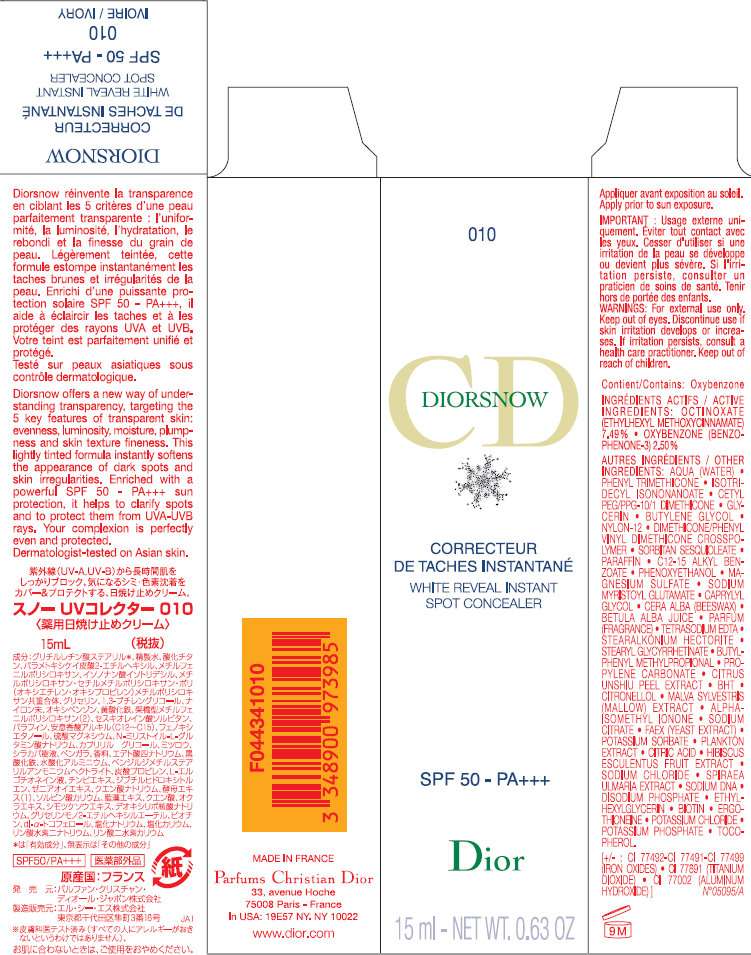
DiorSnow White Reveal Instant Spot Concealer SPF 50 Ivory 010OCTINOXATE EMULSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||