Diltiazem Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILTIAZEM HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- DILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- DILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DILTIAZEM HYDROCHLORIDE DESCRIPTION
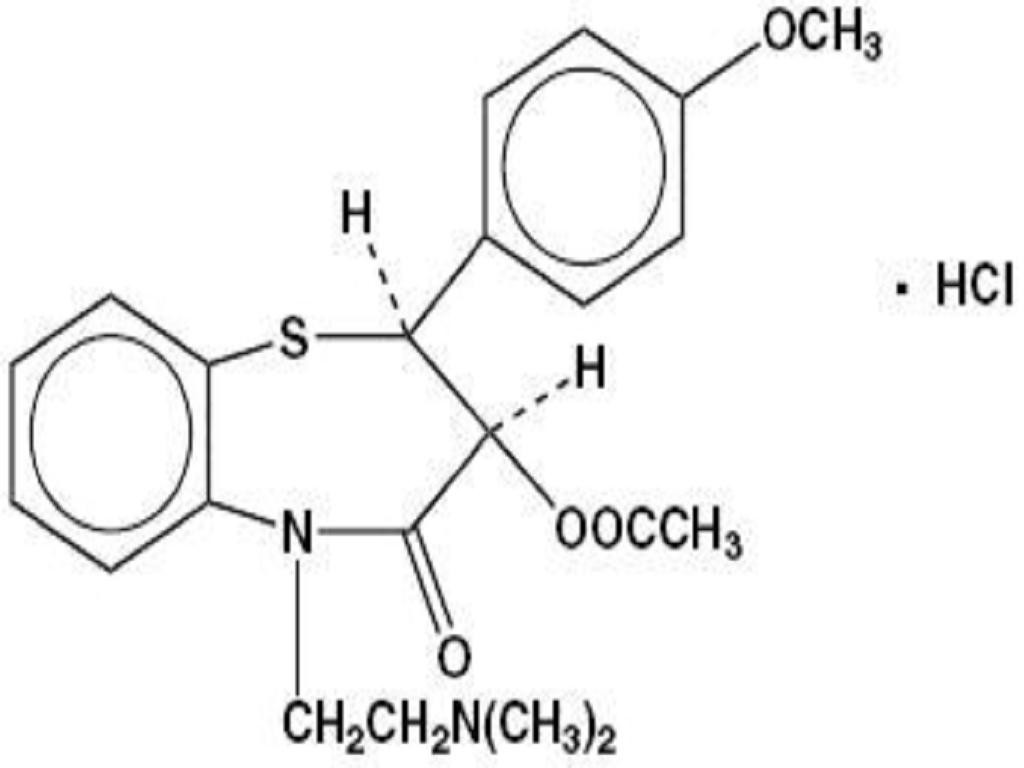
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGYMechanisms of Action
Hypertension
Angina
Hemodynamic and Electrophysiologic Effects
PHARMACODYNAMICS
PharmacodynamicsPHARMACOKINETICS
Pharmacokinetics and MetabolismINDICATIONS & USAGE
INDICATIONS AND USAGEDILTIAZEM HYDROCHLORIDE CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
WARNINGSCardiac Conduction
Congestive Heart Failure
Hypotension
Acute Hepatic Injury
PRECAUTIONS
PRECAUTIONS
GeneralADVERSE REACTIONS
INFORMATION FOR PATIENTS
Information for PatientsDRUG INTERACTIONS
Drug InteractionsWARNINGSWARNINGS
Beta-Blockers
WARNINGS
Cimetidine
Clonidine
Digitalis
WARNINGS
Anesthetics
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Teratogenic EffectsPregnancy Category C
NURSING MOTHERS
PEDIATRIC USE
Pediatric UseDILTIAZEM HYDROCHLORIDE ADVERSE REACTIONS
ADVERSE REACTIONSHypertension
MOST COMMON ADVERSE EVENTS IN DOUBLE-BLIND, PLACEBO-CONTROLLED HYPERTENSION TRIALS
Adverse EventsDiltiazem HydrochloridePlacebo(COSTART Term)Extended-Release Capsules(n = 87)(Once-a-Day Dosage)*# pts (%)n = 303# pts (%)*
Angina
Adverse EventsDiltiazem HydrochloridePlacebo(COSTART Term)Extended-Release Capsulesn = 50(Once-a-Day Dosage)*# pts (%)n = 139# pts (%)*
Infrequent Adverse Events
Hypertension
Angina
OVERDOSAGE
OVERDOSAGE OR EXAGGERATED RESPONSEDOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATIONDosage
Hypertension
Angina
Concomitant Use with Other Cardiovascular Agents
Sublingual Nitroglycerin
Prophylactic Nitrate Therapy
Beta-Blockers
WARNINGSPRECAUTIONS
Antihypertensives
HOW SUPPLIED
STORAGE AND HANDLING
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:AMMONIA
SILICON DIOXIDE
DIBUTYL SEBACATE
D&C YELLOW NO. 10
ETHYLCELLULOSES
FD&C RED NO. 40
FD&C BLUE NO. 1
FD&C BLUE NO. 2
GELATIN
HYPROMELLOSES
MAGNESIUM STEARATE
MALTODEXTRIN
CELLULOSE, MICROCRYSTALLINE
OLEIC ACID
POLYETHYLENE GLYCOL
PROPYLENE GLYCOL
SODIUM LAURYL SULFATE
FERROSOFERRIC OXIDE
TITANIUM DIOXIDE
D&C RED NO. 28
SHELLAC
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Diltiazem HydrochlorideDiltiazem Hydrochloride CAPSULE, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!