Dilantin
FULL PRESCRIBING INFORMATION: CONTENTS*
- DILANTIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DILANTIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- DILANTIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DILANTIN DESCRIPTION
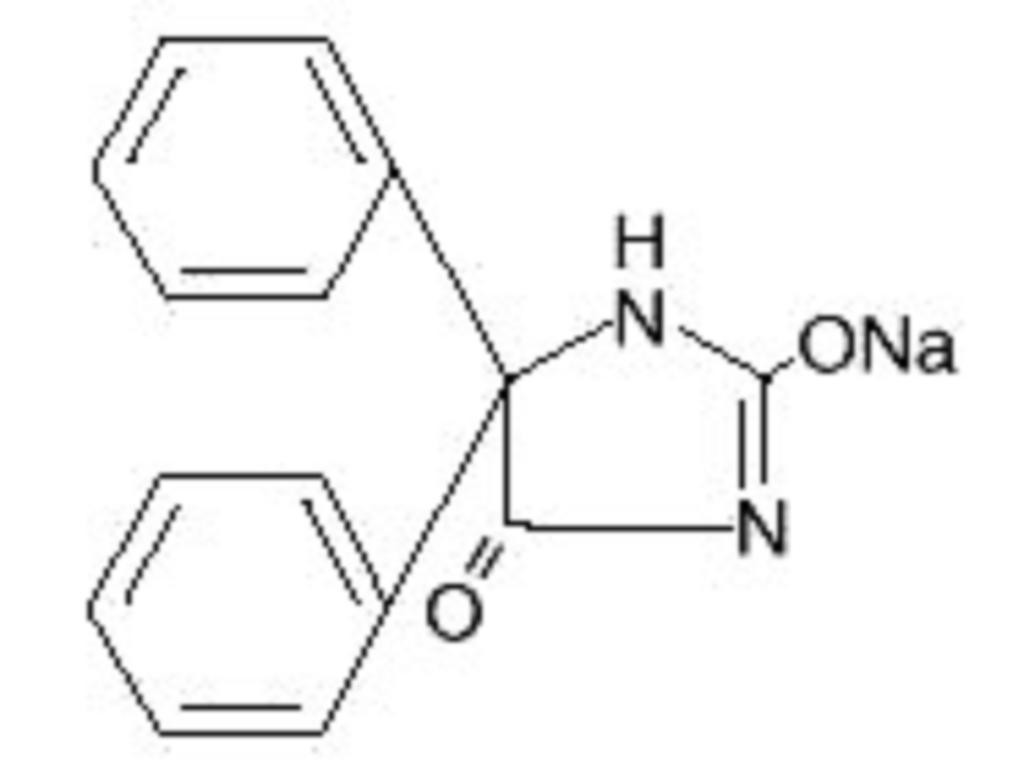
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
DOSAGE AND ADMINISTRATIONCLINICAL PHARMACOLOGY
DILANTIN CONTRAINDICATIONS
WARNINGS
Effects of Abrupt WithdrawalSuicidal Behavior and Ideation
IndicationPlacebo Patients with Events Per 1000 PatientsDrug Patients with Events Per 1000 PatientsRelative Risk: Incidence of Events in Drug Patients/Incidence in Placebo PatientsRisk Difference: Additional Drug Patients with Events Per 1000 Patients
Lymphadenopathy
Effects of Alcohol Use on Phenytoin Serum Levels
Exacerbation of Porphyria
Usage In Pregnancy
Clinical
PRECAUTIONS, Laboratory Tests
Preclinical
Skin reactions
WARNINGS
Anticonvulsant Hypersensitivity Syndrome
PRECAUTIONS
GeneralWARNINGS
WARNINGSADVERSE REACTIONS
CONTRAINDICATIONS
WARNINGS
INFORMATION FOR PATIENTS
PRECAUTIONS: Pregnancy
LABORATORY TESTS
DRUG INTERACTIONS
Drug Enteral Feeding/Nutritional Preparations Interaction
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
WARNINGSPREGNANCY
Pregnancy Category DWARNINGS
NURSING MOTHERS
PEDIATRIC USE
DOSAGE AND ADMINISTRATIONDILANTIN ADVERSE REACTIONS
Central Nervous SystemGastrointestinal System
Integumentary System
PRECAUTIONSWARNINGS
Hemopoietic System
WARNINGS
Connective Tissue System
Immunologic
OVERDOSAGE
Treatment
DOSAGE & ADMINISTRATION
General
Adult Dosage
Divided daily dosage
Once-a-day dosage
Loading dose
Pediatric Dosage
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● How can I watch for early symptoms of suicidal thoughts and actions?
-
● Keep all follow-up visits with your healthcare provider as scheduled.
-
● Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
-
● Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
-
● Birth defects may occur even in children born to women who are not taking any medicines and do not have other risk factors
-
● If you take DILANTIN during pregnancy, your baby is also at risk for bleeding problems right after birth. Your healthcare provider may give you and your baby medicine to prevent this.
-
● All women of child-bearing age should talk to their healthcare provider about using other possible treatments instead of DILANTIN. If the decision is made to use DILANTIN, you should use effective birth control (contraception) unless you are planning to become pregnant.
-
● Tell your healthcare provider right away if you become pregnant while taking DILANTIN. You and your healthcare provider should decide if you will take DILANTIN while you are pregnant.
-
● Pregnancy Registry: If you become pregnant while taking DILANTIN, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
-
● 3.
-
● trouble swallowing or breathing
-
● a skin rash
-
● hives
-
● fever, swollen glands, or sore throat that do not go away or come and go
-
● painful sores in the mouth or around your eyes
-
● yellowing of your skin or eyes
-
● unusual bruising or bleeding
-
● severe fatigue or weakness
-
● severe muscle pain
-
● frequent infections or an infection that does not go away
-
● Call your healthcare provider right away if you have any of the symptoms listed above.
-
● have had an allergic reaction to CEREBYX (fosphenytoin), PEGANONE (ethotoin), or MESANTOIN (mephenytoin).
-
● What should I tell my healthcare provider before taking DILANTIN?
-
● Have or had porphyria
-
● Have or had diabetes
-
● Have or have had depression, mood problems, or suicidal thoughts or behavior
-
● Are pregnant or plan to become pregnant.
-
● If you become pregnant while taking DILANTIN, the level of DILANTIN in your blood may decrease, causing your seizures to become worse. Your healthcare provider may change your dose of DILANTIN.
-
● Are breast feeding or plan to breastfeed. DILANTIN can pass into breast milk. You and your healthcare provider should decide if you will take DILANTIN or breastfeed. You should not do both.
-
● Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
-
● Your healthcare provider may change your dose. Do not change your dose of DILANTIN without talking to your healthcare provider.
-
● DILANTIN can cause overgrowth of your gums. Brushing and flossing your teeth and seeing a dentist regularly while taking DILANTIN can help prevent this.
-
● If you take too much DILANTIN, call your healthcare provider or local Poison Control Center right away.
-
● Do not stop taking DILANTIN without first talking to your healthcare provider. Stopping DILANTIN suddenly can cause serious problems.
-
● What should I avoid while taking DILANTIN?
What is the most important information I should know about DILANTIN?
-
● Call your healthcare provider right away, if you have any of the symptoms listed above.
-
● slurred speech
-
● confusion
-
● dizziness
-
● trouble sleeping
-
● nervousness
-
● tremor
-
● headache
-
● nausea
-
● vomiting
-
● constipation
-
● rash
-
● These are not all the possible side effects of DILANTIN. For more information, ask your healthcare provider or pharmacist.
-
● Store DILANTIN INFATABS at room temperature between 59to 86(15to 30
-
● Store DILANTIN Capsules at room temperature between 68to 77(20to 25in tight, light-resistant containers. Protect from moisture.
-
● Keep DILANTIN and all medicines out of the reach of children.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

DilantinPhenytoin Sodium CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!