Digoxin
FULL PRESCRIBING INFORMATION: CONTENTS*
- DIGOXIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- INDICATIONS & USAGE
- DIGOXIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- PREGNANCY
- NURSING MOTHERS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- GERIATRIC USE
- PEDIATRIC USE
- DIGOXIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
DIGOXIN DESCRIPTION
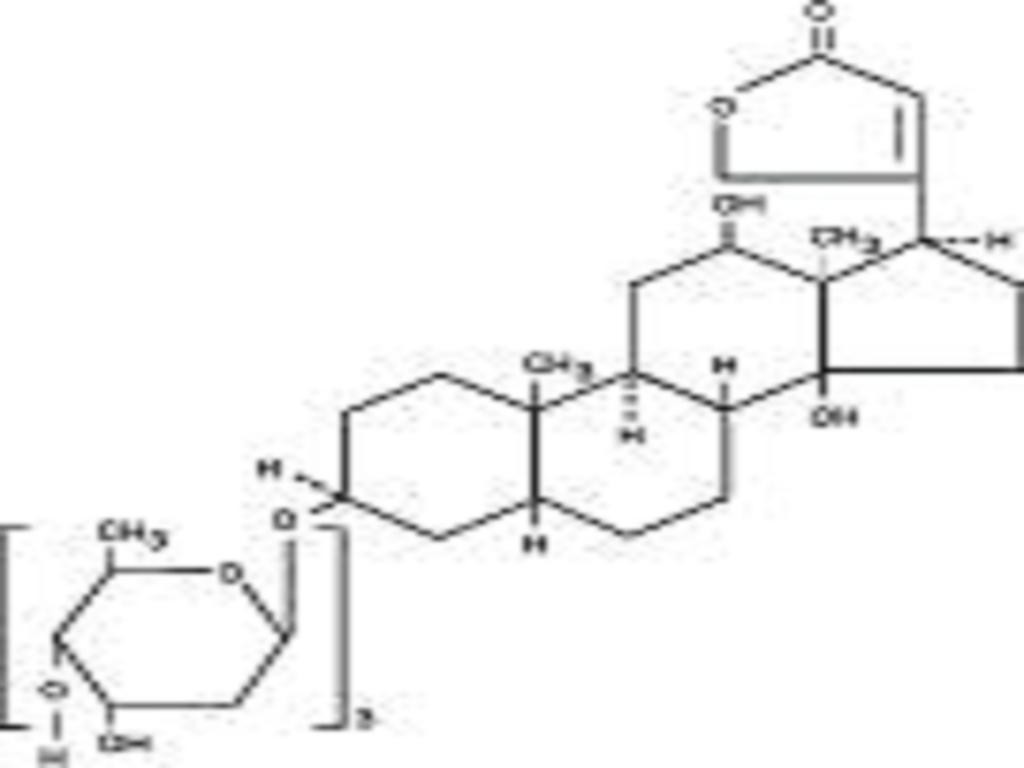
CLINICAL PHARMACOLOGY
Mechanism of ActionPHARMACOKINETICS
Distribution
DOSAGE AND ADMINISTRATION: Serum Digoxin Concentrations
Metabolism
Excretion
Special Populations
DOSAGE AND ADMINISTRATION
Pharmacodynamic and Clinical Effects
Hemodynamic Effects
Chronic Heart Failure
DOSAGE AND ADMINISTRATION
Chronic Atrial Fibrillation
INDICATIONS & USAGE
Heart FailureAtrial Fibrillation
DIGOXIN CONTRAINDICATIONS
WARNINGS
Sinus Node Disease and AV BlockAccessory AV Pathway (Wolff-Parkinson-White Syndrome)
Use in Patients with Preserved Left Ventricular Systolic Function
PRECAUTIONS
Use in Patients with Impaired Renal FunctionDOSAGE AND ADMINISTRATION
Use in Patients with Electrolyte Disorders
Use in Thyroid Disorders and Hypermetabolic States
Use in Patients with Acute Myocardial Infarction
Use During Electrical Cardioversion
Use in Patients with Myocarditis
Use in Patients with Beri Beri Heart Disease
Laboratory Test Monitoring
DOSAGE AND ADMINISTRATION
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY: AbsorptionDue to the considerable variability of these interactions; the dosage of digoxin should be individualized when patients receive these medications concurrently. Furthermore, caution should be exercised when combining digoxin with any drug that may cause a significant deterioration in renal function, since a decline in glomerular filtration or tubular secretion may impair the excretion of digoxin.
DRUG & OR LABORATORY TEST INTERACTIONS
PREGNANCY
Teratogenic Effects: Pregnancy Category CNURSING MOTHERS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
GERIATRIC USE
DOSAGE AND ADMINISTRATIONPEDIATRIC USE
DIGOXIN ADVERSE REACTIONS
Adults: Cardiac
WARNINGSPRECAUTIONS
Gastrointestinal
CNS
Other
Infants and Children
www.fda.gov/medwatch
OVERDOSAGE
Signs and SymptomsADVERSE REACTIONSDOSAGE AND ADMINISTRATION
Adults
ADVERSE REACTIONS
Children
Treatment
WARNINGSPRECAUTIONS: Drug Interactions
Massive Digitalis Overdosage
Administration of Potassium
Administration of Potassium
Massive Digitalis Overdosage
DOSAGE & ADMINISTRATION
GeneralPRECAUTIONS
Serum Digoxin Concentrations
Maintenance Dosing
WARNINGSPRECAUTIONS
Heart Failure: Adults
Rapid Digitalization with a Loading Dose
PRECAUTIONS
CLINICAL PHARMACOLOGY
Maintenance Dosing
Example
Infants and Children
Atrial Fibrillation
Dosage Adjustment When Changing Preparations
CLINICAL PHARMACOLOGY: Pharmacokinetics
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

DigoxinDigoxin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!