Diazepam
DIAZEPAM
FULL PRESCRIBING INFORMATION: CONTENTS*
- DIAZEPAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- DIAZEPAM INDICATIONS AND USAGE
- DIAZEPAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DIAZEPAM ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- DIAZEPAM DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Injection, USP
5 mg/mL
CIV
Fliptop Vial
Rx only
DIAZEPAM DESCRIPTION
Diazepam Injection, USP is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each milliliter (mL) contains 5 mg diazepam; 40% propylene glycol; 10% alcohol; 5% sodium benzoate and benzoic acid added as buffers; and 1.5% benzyl alcohol added as a preservative. pH 6.6 (6.2 to 6.9). Note: Solution may appear colorless to light yellow.
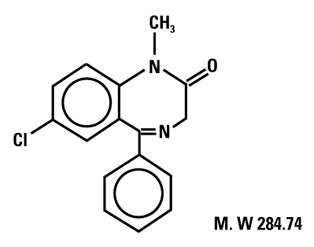
Diazepam is a benzodiazepine derivative chemically designated as 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless crystalline compound, insoluble in water, with the following molecular structure:
CLINICAL PHARMACOLOGY
In animals, diazepam appears to act on parts of the limbic system, the thalamus and hypothalamus, and induces calming effects. Diazepam, unlike chlorpromazine and reserpine, has no demonstrable peripheral autonomic blocking action, nor does it produce extrapyramidal side effects; however, animals treated with diazepam do have a transient ataxia at higher doses. Diazepam was found to have transient cardiovascular depressor effects in dogs. Long-term experiments in rats revealed no disturbances of endocrine function. Injections into animals have produced localized irritation of tissue surrounding injection sites and some thickening of veins after intravenous use.
DIAZEPAM INDICATIONS AND USAGE
Diazepam is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
In acute alcohol withdrawal, diazepam may be useful in the symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
As an adjunct prior to endoscopic procedures if apprehension, anxiety or acute stress reactions are present, and to diminish the patient’s recall of the procedures. (See WARNINGS.)
Diazepam is a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such as inflammation of the muscles or joints, or secondary to trauma); spasticity caused by upper motor neuron disorders (such as cerebral palsy and paraplegia); athetosis; stiff-man syndrome; and tetanus.
Diazepam is a useful adjunct in status epilepticus and severe recurrent convulsive seizures.
Diazepam is a useful premedication (the I.M. route is preferred) for relief of anxiety and tension in patients who are to undergo surgical procedures. Intravenously, prior to cardioversion for the relief of anxiety and tension and to diminish the patient’s recall of the procedure.
DIAZEPAM CONTRAINDICATIONS
Diazepam is contraindicated in patients with a known hypersensitivity to this drug; acute narrow angle glaucoma; and open angle glaucoma unless patients are receiving appropriate therapy.
WARNINGS
When used intravenously, the following procedures should be undertaken to reduce the possibility of venous thrombosis, phlebitis, local irritation, swelling, and, rarely, vascular impairment; the solution should be injected slowly, taking at least one minute for each 5 mg (1 mL) given; do not use small veins, such as those on the dorsum of the hand or wrist; extreme care should be taken to avoid intra-arterial administration or extravasation.
Do not mix or dilute diazepam with other solutions or drugs in syringe or infusion container. If it is not feasible to administer diazepam directly I.V., it may be injected slowly through the infusion tubing as close as possible to the vein insertion.
Extreme care must be used in administering Diazepam Injection, particularly by the I.V. route, to the elderly, to very ill patients and to those with limited pulmonary reserve because of the possibility that apnea and/or cardiac arrest may occur. Concomitant use of barbiturates, alcohol or other central nervous system depressants increases depression with increased risk of apnea. Resuscitative equipment including that necessary to support respiration should be readily available.
When diazepam is used with a narcotic analgesic, the dosage of the narcotic should be reduced by at least one-third and administered in small increments. In some cases the use of a narcotic may not be necessary.
Diazepam Injection should not be administered to patients in shock, coma, or in acute alcoholic intoxication with depression of vital signs. As is true of most CNS-acting drugs, patients receiving diazepam should be cautioned against engaging in hazardous occupations requiring complete mental alertness, such as operating machinery or driving a motor vehicle.
Tonic status epilepticus has been precipitated in patients treated with I.V. diazepam for petit mal status or petit mal variant status.
Usage in Pregnancy:
An increased risk of congenital malformations associated with the use of minor tranquilizers (diazepam, meprobamate and chlordiazepoxide) during the first trimester of pregnancy has been suggested in several studies. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physicians about the desirability of discontinuing the drug.
In humans, measurable amounts of diazepam were found in maternal and cord blood, indicating placental transfer of the drug. Until additional information is available, diazepam injection is not recommended for obstetrical use.
Pediatric Use:
Efficacy and safety of parenteral diazepam has not been established in the neonate (30 days or less of age).
Prolonged central nervous system depression has been observed in neonates, apparently due to inability to biotransform diazepam into inactive metabolites.
In pediatric use, in order to obtain maximal clinical effect with the minimum amount of drug and thus to reduce the risk of hazardous side effects, such as apnea or prolonged periods of somnolence, it is recommended that the drug be given slowly over a three-minute period in a dosage not to exceed 0.25 mg/kg. After an interval of 15 to 30 minutes the initial dosage can be safely repeated. If, however, relief of symptoms is not obtained after a third administration, adjunctive therapy appropriate to the condition being treated is recommended.
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines (see DRUG ABUSE AND DEPENDENCE section).
Benzyl alcohol has been reported to be associated with a fatal gasping syndrome in premature infants.
PRECAUTIONS
Although seizures may be brought under control promptly, a significant proportion of patients experience a return to seizure activity, presumably due to the short-lived effect of diazepam after I.V. administration. The physician should be prepared to re-administer the drug. However, diazepam is not recommended for maintenance, and once seizures are brought under control, consideration should be given to the administration of agents useful in longer term control of seizures.
If diazepam is to be combined with other psychotropic agents or anticonvulsant drugs, careful consideration should be given to the pharmacology of the agents to be employed—particularly with known compounds which may potentiate the action of diazepam, such as phenothiazines, narcotics, barbiturates, MAO inhibitors and other antidepressants. In highly anxious patients with evidence of accompanying depression, particularly those who may have suicidal tendencies, protective measures may be necessary. The usual precautions in treating patients with impaired hepatic function should be observed. Metabolites of diazepam are excreted by the kidney; to avoid their excess accumulation, caution should be exercised in the administration to patients with compromised kidney function.
Since an increase in cough reflex and laryngospasm may occur with peroral endoscopic procedures, the use of a topical anesthetic agent and the availability of necessary countermeasures are recommended.
Until additional information is available, diazepam injection is not recommended for obstetrical use.
Diazepam injection has produced hypotension or muscular weakness in some patients particularly when used with narcotics, barbiturates or alcohol. Lower doses (usually 2 mg to 5 mg) should be used for elderly and debilitated patients.
The clearance of diazepam and certain other benzodiazepines can be delayed in association with cimetidine administration. The clinical significance of this is unclear.
DIAZEPAM ADVERSE REACTIONS
Side effects most commonly reported were drowsiness, fatigue and ataxia; venous thrombosis and phlebitis at the site of injection. Other adverse reactions less frequently reported include: CNS: confusion, depression, dysarthria, headache, hypoactivity, slurred speech, syncope, tremor, vertigo. G.I.: constipation, nausea. G.U.: incontinence, changes in libido, urinary retention. Cardiovascular : bradycardia, cardiovascular collapse, hypotension. EENT: blurred vision, diplopia, nystagmus. Skin: urticaria, skin rash. Other: hiccups, changes in salivation, neutropenia, jaundice. Paradoxical reactions such as acute hyperexcited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported; should these occur, use of the drug should be discontinued. Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during and after diazepam therapy and are of no known significance.
In peroral endoscopic procedures, coughing, depressed respiration, dyspnea, hyperventilation, laryngospasm and pain in throat or chest have been reported.
Because of isolated reports of neutropenia and jaundice, periodic blood counts and liver function tests are advisable during long-term therapy.
DRUG ABUSE AND DEPENDENCE
Diazepam Injection is classified by the Drug Enforcement Administration as a schedule IV controlled substance.
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuance of diazepam. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving diazepam or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
DIAZEPAM DOSAGE AND ADMINISTRATION
Dosage should be individualized for maximum beneficial effect. The usual recommended dose in older children and adults ranges from 2 mg to 20 mg I.M. or I.V., depending on the indication and its severity. In some conditions, e.g., tetanus, larger doses may be required. (See dosage for specific indications.) In acute conditions the injection may be repeated within one hour although an interval of 3 to 4 hours is usually satisfactory. Lower doses (usually 2 mg to 5 mg) and slow increase in dosage should be used for elderly or debilitated patients and when other sedative drugs are administered. (See WARNINGS and ADVERSE REACTIONS.)
For dosage in infants above the age of 30 days and children, see the specific indications below. When intravenous use is indicated, facilities for respiratory assistance should be readily available.
Intramuscular: Diazepam Injection, USP should be injected deeply into the muscle.
Intravenous use: (See WARNINGS, particularly for use in children.) The solution should be injected slowly, taking at least one minute for each 5 mg (1 mL) given. Do not use small veins, such as those on the dorsum of the hand or wrist. Extreme care should be taken to avoid intra-arterial administration or extravasation.
Do not mix or dilute diazepam with other solutions or drugs in syringe or infusion flask. If it is not feasible to administer diazepam directly I.V., it may be injected slowly through the infusion tubing as close as possible to the vein insertion.
Once the acute symptomatology has been properly controlled with diazepam injection, the patient may be placed on oral therapy with diazepam if further treatment is required.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit (see PRECAUTIONS). NOTE: Solution may appear colorless to light yellow.
|
|
USUAL ADULT DOSAGE |
DOSAGE RANGE IN CHILDREN |
|
|
(I.V. administration should be made slowly) |
|
|
Moderate Anxiety Disorders and Symptoms of Anxiety |
2 mg to 5 mg, I.M. or I.V. Repeat in 3 to 4 hours, if necessary. |
|
|
Severe Anxiety Disorders and Symptoms of Anxiety |
5 mg to 10 mg, I.M. or I.V. Repeat in 3 to 4 hours, if necessary. |
|
|
Acute Alcohol Withdrawal: As an aid in symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis. |
10 mg, I.M. or I.V. initially, then 5 mg to 10 mg in 3 to 4 hours, if necessary.
|
|
|
Endoscopic Procedures: Adjunctively, if apprehension, anxiety or acute stress reactions are present prior to endoscopic procedures. Dosage of narcotics should be reduced by at least a third and in some cases may be omitted. See Precautions for peroral procedures. |
Titrate I.V. dosage to desired sedative response, such as slurring of speech, with slow administration immediately prior to the procedure. Generally 10 mg or less is adequate, but up to 20 mg I.V. may be given, particularly when concomitant narcotics are omitted. If I.V. cannot be used, 5 mg to 10 mg I.M. approximately 30 minutes prior to the procedure. |
|
|
Muscle Spasm: Associated with local pathology, cerebral palsy, athetosis, stiff-man syndrome or tetanus. |
5 mg to 10 mg, I.M. or I.V. initially, then 5 mg to 10 mg in 3 to 4 hours, if necessary. For tetanus, larger doses may be required.
|
For tetanus in infants over 30 days of age, 1 mg to 2 mg I.M. or I.V., slowly, repeated every 3 to 4 hours as necessary. In children 5 years or older, 5 mg to 10 mg repeated every 3 to 4 hours may be required to control tetanus spasms. Respiratory assistance should be available. |
|
Status Epilepticus and Severe Recurrent Convulsive Seizures: In the convulsing patient, the I.V. route is by far preferred. This injection should be administered slowly. However, if I.V. administration is impossible, the I.M. route may be used. |
5 mg to 10 mg initially (I.V. preferred). This injection may be repeated if necessary at 10 to 15 minute intervals up to a maximum dose of 30 mg. If necessary, therapy with diazepam may be repeated in 2 to 4 hours; however, residual active metabolites may persist, and readministration should be made with this consideration. Extreme caution must be exercised with individuals with chronic lung disease or unstable cardiovascular status. |
Infants over 30 days of age and children under 5 years, 0.2 mg to 0.5 mg slowly every 2 to 5 minutes up to a maximum of 5 mg (I.V. preferred). Children 5 years or older, 1 mg every 2 to 5 minutes up to a maximum of 10 mg (slow I.V. administration preferred). Repeat in 2 to 4 hours if necessary. EEG monitoring of the seizure may be helpful.
|
|
Preoperative Medication: To relieve anxiety and tension. (If atropine, scopolamine or other premedications are desired, they must be administered in separate syringes.) |
10 mg, I.M. (preferred route), before surgery.
|
|
|
Cardioversion: To relieve anxiety and tension and to reduce recall of procedure. |
5 mg to 15 mg, I.V., within 5 to 10 minutes prior to the procedure. |
|
MANAGEMENT OF OVERDOSAGE
Manifestations of diazepam overdosage include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored, as in all cases of drug overdosage, although, in general, these effects have been minimal. General supportive measures should be employed, along with intravenous fluids, and an adequate airway maintained. Hypotension may be combated by the use of norepinephrine or metaraminol. Dialysis is of limited value.
Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for re-sedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS , WARNINGS , and PRECAUTIONS should be consulted prior to use.
HOW SUPPLIED
Diazepam Injection, USP is supplied as follows:
10 mL multiple dose vials containing 50 mg (5 mg/mL)
Box of 10 NDC 0409-3213-12
Store at 20 to 25ºC (68 to 77ºF). [See USP Controlled Room Temperature.]
Protect from light.
ANIMAL PHARMACOLOGY
Oral LD50 of diazepam is 720 mg/kg in mice and 1240 mg/kg in rats. Intraperitoneal administration of 400 mg/kg to a monkey resulted in death on the sixth day.
Reproduction Studies: A series of rat reproduction studies was performed with diazepam in oral doses of 1, 10, 80 and 100 mg/kg given for periods ranging from 60–228 days prior to mating. At 100 mg/kg there was a decrease in the number of pregnancies and surviving offspring in these rats. These effects may be attributable to prolonged sedative activity, resulting in lack of interest in mating and lessened maternal nursing and care of the young. Neonatal survival of rats at doses lower than 100 mg/kg was within normal limits. Several neonates, both controls and experimentals, in these rat reproduction studies showed skeletal or other defects. Further studies in rats at doses up to and including 80 mg/kg/day did not reveal significant teratological effects on the offspring. Rabbits were maintained on doses of 1, 2, 5 and 8 mg/kg from day 6 through day 18 of gestation. No adverse effects on reproduction and no teratological changes were noted.
Revised: July, 2006
©Hospira 2006 EN-1236 Printed in USA
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
Cardinal Health
Zanesville, OH 43701
IAC61580612
PRINCIPAL DISPLAY PANEL

DiazepamDIAZEPAM INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||