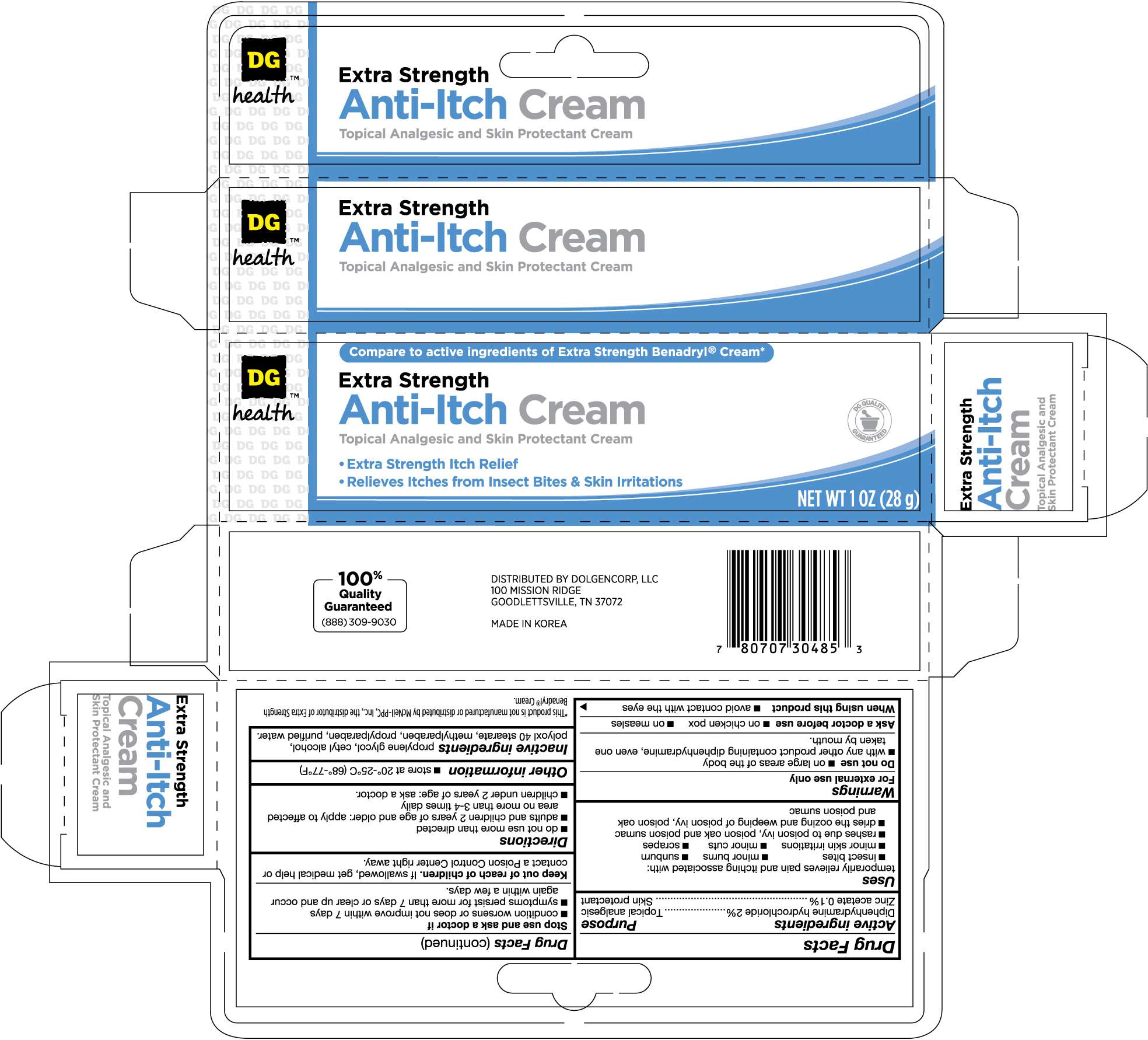
DG HEALTH ANTI ITCH
DOLGENCORP, INC.
UNITED EXCHANGE CORP.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients Purpose
Diphenhydramine Hydrochloride 2% ............................................Topical analgesic
Zinc acetate 0.1% .....................................................................Skin protectant
Purpose
Uses
temporarily relieves pain and itching associated with:
- insect bites
- minor burns
- sunburn
- minor skin irritations
- minor cuts
- scrapes
- rashes due to poison ivy, poison oak and poison sumac
- dries the oozing and weeping of poison ivy, poison oak and poison sumac
Warnings
For external use only
Do not use
- on large areas of the body
- with any other product containing diphenhydramine, even one taken by mouth
Ask a doctor before use
- on chicken pox
- on measles
When using this product
- avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens or does not improve within 7 days
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Uses
Directions
- do not use more than directed
- adults and children 2 years of age and older: apply to affected area no more than 3-4 times daily
- children under 2 years of age: ask a doctor
Other information
- store at 20o to 25oC (68o-77oF)
Inactive ingredients
propylene glycol, cetyl alcohol, polyoxyl 40 stearate, methylparaben, propylparaben, purified water.
Distributed By Dolgencorp, LLC
100 Mission Ridge
Goodlettsville, TN 37072
Made in Korea
 Enter section text here
Enter section text here
DG HEALTH ANTI ITCHDIPHENHYDRAMINE HYDROCHLORIDE, ZINC ACETATE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||