DermaNIC
DermaNic with Quadracin™ Plus CitraFolic, AminoFerr, and Zinc-NACx
FULL PRESCRIBING INFORMATION: CONTENTS*
- DERMANIC DESCRIPTION
- ALLERGY STATEMENT
- DERMANIC CONTRAINDICATIONS
- INTERACTIONS
- WARNINGS
- PRECAUTIONS
- DERMANIC ADVERSE REACTIONS
- PHARMACOLOGY
- PHARMACOLOGICAL COFACTOR-INGREDIENTS
- MECHANISM OF ACTION
- FOLATE (R) REGULATION
- PATIENT INFORMATION
- PREGNANCY AND NURSING MOTHERS
- DERMANIC INDICATIONS AND USAGE
- DERMANIC DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
- REFERENCES
- PRINCIPAL DISPLAY PANEL - 60 Tablet Bottle Label
FULL PRESCRIBING INFORMATION
PRESCRIPTION (Rx)-DIETARY SUPPLEMENT
MULTIPHASIC TABLETS
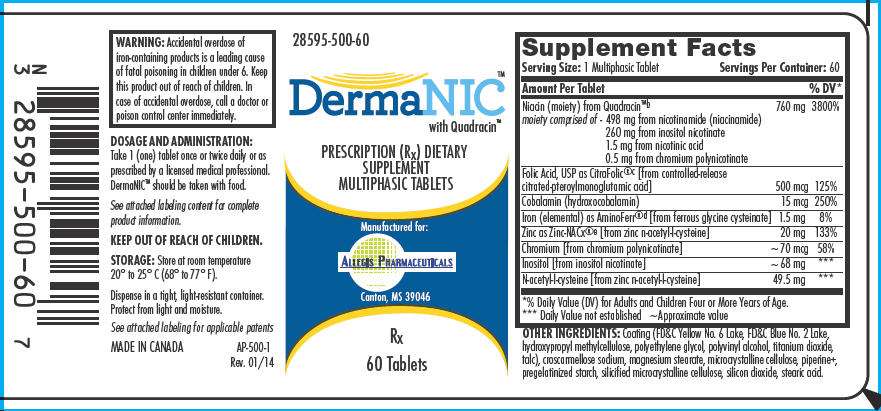
PRODUCT CODE: ++28595-500-60
DERMANIC DESCRIPTION
DermaNIC™ is an orally administered, folate-containing prescription (Rx) dietary supplement for the clinical dietary management of suboptimal niacin and zinc levels associated with acne and/or acne therapy. DermaNIC™ may be administered as adjunctive niacin-folate therapy to provide a protective effect in reducing the risk of hyperhomocysteinemia and/or pellegra in patients undergoing acne therapy or may be administered as monotherapy for patients who are in need of advanced niacin and zinc supplementation as determined by a licensed medical practitioner.2,3,20,22,23 DermaNIC™ is not a drug, but may be prescribed along with acne medications for concomitant care.3 The ultimate goal of acne treatment is to address as many of the pathogenic factors of acne as possible while minimizing side effects.
DermaNIC™ is formulated with niacin and zinc, which support healthful methylation biochemistry and have anti-inflammatory effects via preservation of intracellular coenzyme homeostasis.21 Furthermore, natural ingredients have been added to DermaNIC™ that combine anti-inflammatory and antimicrobial properties along with inhibiting effects on sebum production.22,24-26 Lastly, antibiotic use, which is common in acne patients, can lower the levels of B vitamins that are essential for methylation biochemistry.18 Specifically, studies have shown that acne patients on isotretinoin therapy have decreased folate levels and increased levels of homocysteine.19
| Supplement Facts | ||||
|---|---|---|---|---|
| Serving Size: 1 Multiphasic Tablet | Servings Per Container: 60 | |||
| Amount Per Tablet | % DV |
|||
| Niacin (moiety) from Quadracin™ |
760 mg | 3800% | ||
| moiety comprised of - | 498 mg from nicotinamide (niacinamide) 260 mg from inositol nicotinate 1.5 mg from nicotinic acid 0.5 mg from chromium polynicotinate |
|||
| Folic Acid, USP as CitraFolic®
|
500 mcg | 125% | ||
| Cobalamin (hydroxocobalamin) | 15 mcg | 250% | ||
| Iron (elemental) as AminoFerr®
|
1.5 mg | 8% | ||
| Zinc as Zinc-NACx®
|
20 mg | 133% | ||
| Chromium [from chromium polynicotinate] | ~70 mcg | 58% | ||
| Inositol [from inositol nicotinate] | ~68 mg |
 |
||
| N-acetyl-l-cysteine [from zinc n-acetyl-l-cysteine] | 49.5 mg |
 |
||
OTHER INGREDIENTS
Coating (FD&C Yellow No. 6 Lake, FD&C Blue No. 2 Lake, hydroxypropyl methylcellulose, polyethylene glycol, polyvinyl alcohol, titanium dioxide, talc), croscarmellose sodium, magnesium stearate, microcrystalline cellulose, piperine
This product contains FD&C Yellow #6 Lake.
ALLERGY STATEMENT
This product has been manufactured in a facility that also manufactures products containing tree nuts, peanuts, fish, egg, wheat, milk, soy and shellfish. Individuals with allergic tendencies to these substances should use discretion.
DERMANIC CONTRAINDICATIONS
DermaNIC™ is contraindicated in patients with a known hypersensitivity to any of the components contained in this product. DermaNIC™ is contraindicated for individuals with conditions for which any of the DermaNIC™ ingredients are contraindicated.
INTERACTIONS
Talk to your healthcare practitioner and/or pharmacist before taking or using any prescription or over-the-counter medicines or herbal/health supplements alongside DermaNIC™.
This product should not be taken at the same time as tetracycline because it may interfere with the absorption and effectiveness of the antibiotic. This product should be taken at different times of the day, at least two hours apart, from tetracycline.
WARNINGS
This product contains iron.
Warning: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Extreme caution should be used when prescribing this product to patients with a history of liver disease, jaundice, diabetes and/or kidney disease. These patients, as well as patients with a history of heavy use of alcohol, gallbladder disease, gout, and/or stomach ulcers, should be monitored closely. Abnormal liver functions tests have been reported in persons taking high doses of niacin. Patients with coronary artery disease or unstable angina should not take niacin without their licensed medical practitioner's supervision, as large doses can increase the risk of heart rhythm problems. Caution is also advised in patients with low blood pressure as niacin may cause a dangerous drop in blood pressure. Niacin can be toxic to the liver at high doses. Do not exceed 3 grams per day of nicotinamide.1 Do not use other niacin-containing products while taking this product unless under the supervision of a licensed healthcare practitioner. This product is not formulated or intended to be used to treat hyperlipidemias. This product contains four different forms of niacin as Quadracin™, with the majority of the niacin being supplied in slowly metabolized forms, such as nicotinamide and inositol nicotinate. Nicotinamide does not have the same lipid modifying effects as nicotinic acid.
Caution is recommended in patients taking anticonvulsant medications as folate may interfere with anticonvulsant medication, and may lower seizure threshold. Furthermore, it has been reported that anticonvulsant medications interfere with folate metabolism, but the exact action is unclear; therefore caution is recommended with patients in this therapeutic group.
Patients undergoing cancer treatment should consult their licensed medical practitioner for advice. Before having surgery, tell your licensed medical practitioner that you are taking this product.
PRECAUTIONS
Folate alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folate in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations progress.
DERMANIC ADVERSE REACTIONS
"Niacin flush" is a burning, tingling sensation in the face and chest, and red or flushed skin and is associated with rapidly metabolized niacin
PHARMACOLOGY
NIACIN
Niacin, or vitamin B3, is a water-soluble vitamin absorbed in the stomach and upper small intestine, and refers to both nicotinic acid and niacinamide. Niacin circulates in the plasma as nicotinic acid and nicotinamide. It is biosynthetically converted into nicotinamide adenine dinucleotide (NAD+) and the phosphorylated dinucleotide nicotinamide adenine dinucleotide phosphate (NADP+). NAD and NADP are coenzymes in a wide variety of enzymatic oxidation-reduction reactions. The antioxidant enzyme glucose-6-phosphate dehydrogenase (G6PD) maintains the level of NADPH which is important for glutathione production. However, G6PD activity has been shown to be low in some acne patients.22 DermaNIC™ provides niacin as Quadracin™ which contains:
NICOTINAMIDE
Nicotinamide is the amide derivative of nicotinic acid, and is provided as niacinamide. In vivo, nicotinamide is incorporate into nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). Nicotinamide has been shown to have anti-inflammatory activities, and has been shown to inhibit lipopolysaccharide-induced TNF-alpha in a number of animal studies. It is thought that this inhibition of TNF-alpha is mediated via inhibition, at the gene transcription level, of NF-Kappa B, which in turn inhibits TNF-alpha. Nicotinamide has also been shown to decrease the production of IL-12 and TNF-alpha in cultures of whole blood from prediabetic and diabetic subjects and also in healthy subjects.
NICOTINIC ACID
Nicotinic acid is an essential dietary constituent, the lack of which leads to pellagra, a condition characterized by an erythematous skin eruption as well as gastrointestinal and neurological symptoms. Nicotinic acid is converted to nicotinamide in vivo. Recent studies suggest that nicotinic acid may inhibit vascular oxidative stress, redox-sensitive genes and monocyte adhesion to human aortic endothelial cells; implying that nicotinic acid inhibits vascular inflammation and has antiatherosclerotic properties independent of, and in addition to, its lipid-modulating effects. Studies have shown that the cutaneous vasodilation nicotinic acid induces - resulting in the so-called "niacin flush" that is harmless but causes some patients discomfort and prevents them from continuing to use nicotinic acid, likely subsides after a couple of doses and/or continued use. Also included in this formulation are two niacin derivative conjugates: 1) INOSITOL NICOTINATE - Inositol nicotinate is a conjugated derivative of nicotinic acid that appears to be slowly metabolized, and therefore is less likely to cause flushing while still delivering the nicotinic acid moiety with the added benefit of inositol; and 2) CHROMIUM POLYNICOTINATE - Chromium polynicotinate is another nicotinic acid derivative conjugate that delivers the nicotinic acid moiety with the added benefit of chromium.
ZINC
The zinc in this product is supplied as Zinc-NACx®, which also provides the glutathione precursor n-acetyl-cysteine (NAC). Zinc plays a role in the folate cycle via the zinc metalloenzyme betaine homocysteine methyl transferase (BHMT). In addition, the antioxidant enzyme superoxide dismutase (SOD) requires zinc as a cofactor. Zinc acts on inflammatory cells, especially granulocytes.2 Studies show that oxidative stress exists in acne patients.21 Zinc levels have been reported to be low in patients with acne.
PHARMACOLOGICAL COFACTOR-INGREDIENTS
IRON
DermaNIC™ supplies iron as pure amino acid iron-chelate, which provides pure elemental iron - an essential component in the formation of hemoglobin. Iron therapy is necessary in advanced folate supplementation due to interference between iron and folate metabolism.6 Sufficient amounts are required for effective erythropoiesis. The selection of a non-heme form of supplemental iron is also important because the heme carrier protein (HCP) has been demonstrated also to be a proton coupled folate transporter (PCFT).6 As a result, when dietary folate intake is high, as would be with the administration of DermaNIC™, heme iron transport can be sacrificed, leading to potential iron deficiency. Co-administering non-heme iron compensates for these potential metabolic imbalances. This proprietary form of iron is protected under US Patent No. 7,341,708.
COBALAMIN
Cobalamin is required for two important reactions: the conversion of methylmalonyl CoA to succinyl CoA, a Krebs cycle intermediate, and the conversion of homocysteine to methionine, a reaction in which the methyl group of methyltetrahydrofolate is donated to remethylate homocysteine.16 Many factors contribute to the cobalamin deficiency including diet, gastrointestinal pathology, autoimmune disease and medications.
BIOAVAILABILITY ENHANCER
Piperine is an alkaloid found naturally in plants, and may have bioavailability-enhancing activity for some nutritional substances and for some drugs.7
FOLATE
Folates are best known for reducing the incidence of fetal neural tube defects (NTDs).24-26 NTDs are congenital malformations produced by failure of the neural tube to form and close properly during embryonic development.17,25 During the first four weeks of pregnancy - when many women do not even realize that they have conceived, adequate maternal folate intake is essential to reduce the risk of NTDs. As the postnatal period approaches there is increased demand again for folate regardless of lactation status. Folate is involved in transformylation and methylation metabolism as well as - indirectly, succinylation metabolism (through the "methyl trap" hypothesis). Folate plays a central role in the formation of nucleic acid precursors, such as thymidylic acid and purine nucleotides, which are essential for nucleic acid synthesis and cell division. IOM/NAS (1998) noted that the evidence for a protective effect from folate supplements is much stronger than that for food folate.17 Other dietary ingredients are added to folate as cofactors, coenzymes and co-metabolites; in studies by Czeizel and Dudas (1992) and Berry et al. (1999), factors other than folate may affect the magnitude of risk reduction or participate in a co-protective effect with folate. The need for folate for methyl group biosynthesis may also increase with high niacin intake. The major pathway of metabolism of nicotinamide is by methylation in the liver to form N-methylnicotinamide via reaction with methionine (as a methyl donor) and ATP. As a result, high levels of niacin may interfere with the metabolism of methionine, which could lead to hyperhomocysteinemia. Increased folate intake may also confer a protective effect against folate depletion and subsequent hyperhomocysteinemia in acne patients taking antibiotics.18-20 Certain antibiotics may affect the absorption and metabolism of folate. Isotretinoin, for example, has been shown to decrease serum folic acid levels.18 Furthermore, studies have reported elevated plasma homocysteine in patients on isotretinoin as quickly as 45 days after beginning therapy.19 Isotretinoin may inhibit the activity of the cystathionine-beta-synthase enzyme, thereby impairing the conversion of homocysteine to cystathionine in the transsulfuration pathway. Furthermore, one of the most common antibiotic treatments for acne includes the Sulfa antibiotics - such as cotrimoxazole, that inhibits folic acid synthesis in bacteria. This highlights the importance of folic acid, or folate, rescue therapy as an adjunctive modality to be used in the treatment of acne alongside traditional acne drug therapy. Additionally, serum folate levels were decreased in post adolescent acne patients.23
The present form of folate in this product is:
FOLIC ACID as CitraFolic®
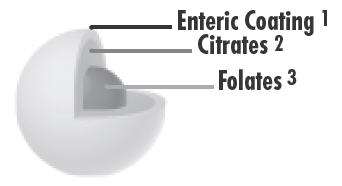
- Enteric coating creates an initial delayed release of approximately 20 minutes, non-pH-dependant, in order to aid in reducing first pass metabolism degradation, high-acidity environmental exposure and/or oxidation factors.
- Citrates are dispersed in a 2:1 ratio of sodium citrate to citric acid in order to achieve optimum pH for folic acid dissolution as per USP specifications.
- Folates are dispersed amongst the citrates in a 1:3 ratio of folic acid to citrates in order to reach maximum dissolution potential in variant mediums.
Note: The total folate-citrate matrix reaches 75% dissolution between the first 20 to 45 minutes, with over 90% folate dissolution achieved - as per USP, within 60 minutes.
MECHANISM OF ACTION
NIACIN is an essential coenzyme in a wide variety of enzymatic oxidation-reduction reactions.24-26 ZINC is required for a number of immune functions, including T-lymphocyte activity. Zinc supplementation can restore impaired immune function in those with zinc deficiency, as found in malabsorption syndromes and acrodermatitis enteropathica.26 IRON is necessary for the production of hemoglobin. Iron-deficiency can lead to decreased production of hemoglobin and a microcytic, hypochromic anemia and/or megaloblastic anemia.26 FOLATE is essential for the production of certain coenzymes in many metabolic systems such as purine and pyrimidine synthesis. It is also essential in the synthesis and maintenance of nucleoprotein in erythropoiesis. It also promotes white blood cell (WBC) and platelet production in folate-deficiency anemia. Folate is associated with methylation and transformylation biochemistry.16,24-26
FOLATE (R) REGULATION
The Federal Register Notices from 1971 to 1973 established that increased folate was proper therapy in megaloblastic anemias of tropical and nontropical sprue, nutritional origin, pregnancy, infancy and childhood.10-13 Folate metabolism can be affected by malabsorption issues which differ widely among population groups. The March 5, 1996 Federal Register Notice (61 FR 8760) states that "The agency concluded that the scientific literature did not support the superiority of any one source of folate over others, and that the data were insufficient to provide a basis for stating that a specific amount of folate is more effective than another amount."16 The actual amount and source of folate require a licensed medical practitioner's supervision to achieve a satisfactory maintenance level, and may exceed the 0.8 mg UL. The Federal Register Notice of August 2, 1973 (38 FR 20750) specifically states that "dietary supplement preparations are available without a prescription (21 CFR 121.1134). Levels higher than dietary supplement amounts are available only with a prescription. Oral preparations supplying more than 0.8 mg of folate per dosage unit would be restricted to prescription dispensing and that a dietary supplement furnishing 0.8 mg could be prescribed when a maintenance level of 0.8 mg per day was indicated. When clinical symptoms have subsided and the blood picture and/or CSF folate levels have become normal, a maintenance level should be used. Patients should be kept under close supervision and adjustment of the maintenance level made if relapse appears imminent. In the presence of alcoholism, hemolytic anemia, anticonvulsant therapy, or chronic infection, the maintenance level may need to be increased."11 However, once the level of active folate exceeds 0.8 mg - as prescribed dosages, then the product is no longer a medical food but a prescription dietary supplement regardless of pregnancy/lactation status in spite of the fact that folic acid - including reduced forms, may be added to medical foods as defined in section 5(b)(3) of the Orphan Drug Act (21 USC 360ee(b)(3)), or to food (21 CFR 172.345).14-15 In the Letter Regarding Dietary Supplement Health Claim for Folic Acid, Vitamin B6, and Vitamin B12 and Vascular Disease (Docket No. 99P-3029) dated November 28, 2000, FDA wrote "... high intakes of folate may partially and temporarily correct pernicious anemia while the neurological damage of vitamin B12 deficiency progresses. IOM/NAS (1998) set the UL for all adults of 1 mg per day because of devastating and irreversible neurological consequences of vitamin B12 deficiency, the data suggesting that pernicious anemia may develop at a younger age in some racial or ethnic groups, and the uncertainty about the extent of the occurrence of vitamin B12 deficiency in younger age groups (IOM/NAS, 1998)."16 Summary: This product is a dietary supplement product that - due to advanced folate levels, requires administration under the care of a licensed medical practitioner, and the most appropriate way to do that is to provide the product as prescription for pedigree reporting and safety monitoring. The ingredients, indication or claims of this product are not to be construed to be drug claims.
PATIENT INFORMATION
DermaNIC™ is a prescription dietary supplement to be used only under licensed medical supervision. Your licensed medical practitioner may choose to prescribe DermaNIC™ along with other medications.
PREGNANCY AND NURSING MOTHERS
DermaNIC™ contains a high dose of niacin and is NOT recommended for pregnant and/or lactating women. However, DermaNIC™ can be administered to women of childbearing age.
DERMANIC INDICATIONS AND USAGE
DermaNIC™ is indicated for the distinct nutritional requirements of individuals undergoing acne therapy who require advanced niacin and/or zinc supplementation. DermaNIC™ is not a drug, but may be used as monotherapy
DERMANIC DOSAGE AND ADMINISTRATION
Take 1 (one) tablet once or twice daily or as prescribed by a licensed medical practitioner
HOW SUPPLIED
DermaNIC™ is supplied as a scored, beige-colored, oval tablet debossed with "044" on one side, in bottles of 60 tablets, (Product Code
STORAGE
Store at room temperature 20° to 25° C (68° to 77° F). Dispense in a tight, light-resistant container. Protect from light and moisture.
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
MADE IN CANADA.
Rx
MANUFACTURED FOR: Allegis Pharmaceuticals, LLC, Canton, MS 39046.
PATENTS: US Patent Nos. 7,341,708, 5,536,506, 5,744,161, 5,972,382; and 6,054,585.
Other patent applications pending.
TRADEMARKS: DermaNIC™ is a trademark of Allegis Pharmaceuticals, LLC (Canton, MS, USA). DermaNIC™ was developed by Captiva Pharma, LLC (Fort Myers, Florida, USA). Zinc-NACx® and CitraFolic® are registered trademarks of Via Naturally, LLC (Fort Myers, Florida, USA). AminoFerr® is a registered trademark of Viva Pharmaceuticals (Richmond, BC, Canada). Quadracin™ is a use-trademark of Captiva Pharma, LLC.
REVISION: January 2014
REFERENCES
- Knip M, Douek IF, Moore WP, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000 Nov;43(11):1337-45.
- Fivenson DP. The mechanisms of action of nicotinamide and zinc in inflammatory skin disease. Cutis. 2006 Jan;77(1 Suppl):5-10.
- Alexis AF. Clinical considerations on the use of concomitant therapy in the treatment of acne. J Dermatol Treat. 2008;19:199–209.
- Hoag SW, Ramachandruni H, Shangraw RF. Failure of prescription prenatal vitamin products to meet USP standards for folic acid dissolution. J Am Pharm Assoc (Wash). 1997
Jul-Aug;NS37(4):397-400. - Younis IR, Stamatakis MK, Callery PS, et al. Influence of pH on the dissolution of folic acid supplements. Int J Pharm. 2009 Feb 9;367(1-2):97-102.
- Laftah A., Latunde-Dada G., Fakih S, et al. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). British Journal of Nutrition.
2009;101:1150–1156. - Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food SciNutr. 2007;47(8):735-48.
- Hallert C, Tobiasson P, Walan A. Serum folate determinations in tracing adult coeliacs. Scand J Gastroenterol. 1981;16:263-67.
- Wu A, Chanarin I, Levi AJ. Macrocytosis of chronic alcoholism. The Lancet. 1974;1:829-31.
- Federal Register Notice of April 9, 1971 (36 FR 6843).
- Federal Register Notice of August 2, 1973 (38 FR 20750).
- Federal Register Notice of October 17, 1980 (45 FR 69043 at 69044).
- Federal Register Notice of March 5, 1996 (61 FR 8760).
- Code of Federal Regulations Title 21 Section 172.345.
- Code of Federal Regulations Title 21 Section 101.9(j)(8).
- Letter Regarding Dietary Supplement Health Claim for Folic Acid, Vitamin B6, and Vitamin B12 and Vascular Disease (Docket No. 99P-3029). November 28, 2000.
- Letter Regarding Dietary Supplement Health Claim for Folic Acid With Respect to Neural Tube Defects (Docket No. 91N-100H). October 10, 2000.
- Javanbakht AM, Pour HM, Tarrahic MJ. Effects of oral isotretinoin on serum folic acid levels. J Drugs Dermatol. 2012 Sep;11(9):e23-4.
- Schulpis KH, Karikas GA, Georgala S, et al. Elevated plasma homocysteine levels in patients on isotretinoin therapy for cystic acne. Int J Dermatol. 2001 Jan;40(1):33-6.
- Clinical considerations on the use of concomitant therapy in the treatment of acne. J Dermatol Treat. 2008;19:199–209.
- Ozuguz P, DogrukKacar S, Ekiz O, et al. Evaluation of serum vitamins A and E and zinc levels according to the severity of acne vulgaris. CutanOculToxicol. 2013 Jul 5.
- Aracan et al. Oxidative Stress in Patients With Acne Vulgaris. Mediators Inflamm. 2005 December 14; 2005(6): 380–384.
- Balta et al. Nutritional anemia in reproductive age women with post adolescent acne. CutanOculToxicol. 2013 Sep;32(3):200-3. doi: 10.3109/15569527.2012.751393. Epub 2013 Jan 25.
- Hendler SS, Rorvik D, eds. PDR for Nutritional Supplements. Montvale, NJ: Thomson Healthcare;2001.
- Hendler SS, Rorvik D. PDR for Nutritional Supplements. 2nd ed. Montvale, NJ. Physicians' Desk Reference Inc;2008.
- Bendich A, Deckelbaum R. Preventive Nutrition: The Comprehensive Guide for Health Professionals. 2009.
PRINCIPAL DISPLAY PANEL - 60 Tablet Bottle Label
28595-500-60
DermaNIC™
with Quadracin™
PRESCRIPTION (Rx) DIETARY
SUPPLEMENT
MULTIPHASIC TABLETS
Manufactured for:
ALLEGIS PHARMACEUTICALS
Canton, MS 39046
Rx
60 Tablets
DermaNICNIACINAMIDE, INOSITOL NIACINATE, NIACIN, CHROMIUM NICOTINATE, FOLIC ACID, HYDROXOCOBALAMIN, FERROUS CYSTEINE GLYCINATE, and ACETYLCYSTEINE ZINC TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||