DENDRID
DENDRID®(idoxuridine)Sterile Ophthalmic Solution
FULL PRESCRIBING INFORMATION: CONTENTS*
- DENDRID DESCRIPTION
- CLINICAL PHARMACOLOGY
- DENDRID INDICATIONS AND USAGE
- CONTRAINDICATION
- PRECAUTIONS
- DENDRID ADVERSE REACTIONS
- DENDRID DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
FULL PRESCRIBING INFORMATION
DESCRIPTION
Dendrid® (idoxuridine) is an antiviral chemotherapeutic agent prepared in a sterile buffered isotonic solution. The active ingredient is represented by the chemical structure:
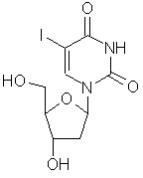
Established name:
Idoxuridine
Chemical name:
Uridine, 2’-deoxy-5-iodo-
Each ml contains: Active: Idoxuridine 0.1%. Preservative: Benzalkonium Chloride 0.01%. Inactive: Boric Acid, Edetate Disodium, Sodium Hydroxide and/or Hydrochloric Acid (to adjust pH), Purified Water. DM-01
CLINICAL PHARMACOLOGY
Herpes simplex virus utilizes thymidine in the synthesis of deoxyribonucleic acid (DNA), a metabolite necessary for reproduction. Idoxuridine is identical in chemical structure to thymidine except that the 5-methyl group is replaced by iodine. When idoxuridine is substituted for thymidine in DNA, the cell is unable to utilize the DNA and reproduction ceases.
INDICATIONS AND USAGE
For the treatment of keratitis caused by the virus of herpes simplex.
CONTRAINDICATION
Hypersensitivity to the active ingredient or any other component of this drug.
PRECAUTIONS
For topical use only. Do not exceed the frequency or duration of recommended dosage. The incidence of some adverse reactions increases with prolonged use. Idoxuridine is not effective in corneal inflammations following herpes simplex keratitis in which the virus is not present. Some strains of herpes simplex appear resistant to the action of Idoxuridine.
USAGE IN PREGNANCY
Idoxuridine should be administered with caution in pregnancy or in women of childbearing potential. Idoxuridine has been reported to cross the placental barrier and to produce fetal malformations in rabbits when administered topically to the eyes of pregnant females in doses similar to those used clinically. Idoxuridine has also been reported to produce fetal malformations in the rat after intraperitoneal and oral administration and in the mouse after subcutaneous administration. Nursing should not be performed while a patient is undergoing IDU treatment as the drug and metabolites may be excreted in human milk.
MUTAGENIC POTENTIAL
Idoxuridine has been reported to cause chromosome aberrations in mice and to be mutagenic in mammalian cells in culture (e.g., diploid human lymphoblasts and mouse lymphoma cells). Drosophila melanogaster and in a host-mediated assay system utilizing mammalian cells.
ONCOGENIC POTENTIAL
The studies performed to date on idoxuridine are inadequate for assessment of carcinogenicity. This cytotoxic drug should be regarded as being potentially carcinogenic. It can inhibit DNA synthesis or function and is incorporated into the DNA of mammalian cells as well as into this genome of DNA viruses. Indoxuridine has been reported to induce RNA tumor virus (type C particles) production from virus negative mouse cells. The degree of oncogenic activity of idoxuridine induced oncornaviruses has not been documented. However, several idoxuridine activated oncornaviruses have caused in vitro cell transformation and induction of specific neoplasms (lymphatic leukemias and carcinomas) upon inoculation into syngenic mice.
ADVERSE REACTIONS
Acute ocular irritation including burning, corneal stippling, vascularization, and clouding has been reported. Following prolonged use of idoxuridine, ocular irritation characterized by follicular conjunctivitis, blepharitis with punctal swelling, bulbar conjunctival hyperemia, and corneal epithelial staining has also been reported. In either case, the drug should be discontinued.
DOSAGE AND ADMINISTRATION
Instill one drop in the affected eye(s) every hour. In acute herpes, dosage may be tapered through every two hours to four times daily prior to discontinuation (treatment should be continued for at least seven days).
HOW SUPPLIED
In a 15 ml plastic Drop-Tainer® dispenser.
NDC 0065-0029-15
STORAGE: Between 36°-80°F. Protect from light.
REFERENCES
- hoi, M., and Y. Ishll. Teratogenicity of IDU Ophthalmic. Acta Soc. Ophthal. Sup. 76:52-54 (summary in English) (p. 150), 1972.
- hoi, M., J. W. Getter, N. Kaneko, Y. Ishll, R. M. Ramer, and A. R. Gasset. Teratogenicities of ophthalmic drugs. L Antiviral ophthalmic drugs. Arch. Ophthal. 93:46-51, 1975.
- Perry, D. H., D. M. Albert and T. Amemyia. Ocular defects in newborn rats treated with 5-iodo-deoxyuridine (IUDR). Proc. Soc. Exp. Biol. and Med. 142:1272-1276, 1973.
- Data on file with IND filed by Smith, Kline and French.
- Calabresl, Paul. Current status of clinical investigations with 6-Azauridine, 5-iodo-2’-deoxyuridine, and related derivatives. Cancer Research. 23:1260-1267, Sept. 1963.
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 USA
298351
Printed in USA
DENDRIDidoxuridine SOLUTION/ DROPS
| |||||||||||||||||||||||||||||||||||||||||||||||||||