Delsym
Delsym Children's NIGHT TIME COUGH & COLD
FULL PRESCRIBING INFORMATION: CONTENTS*
- Delsym Uses
- Warnings
- Directions
- Delsym Other information
- Inactive ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 118 mL Carton
FULL PRESCRIBING INFORMATION
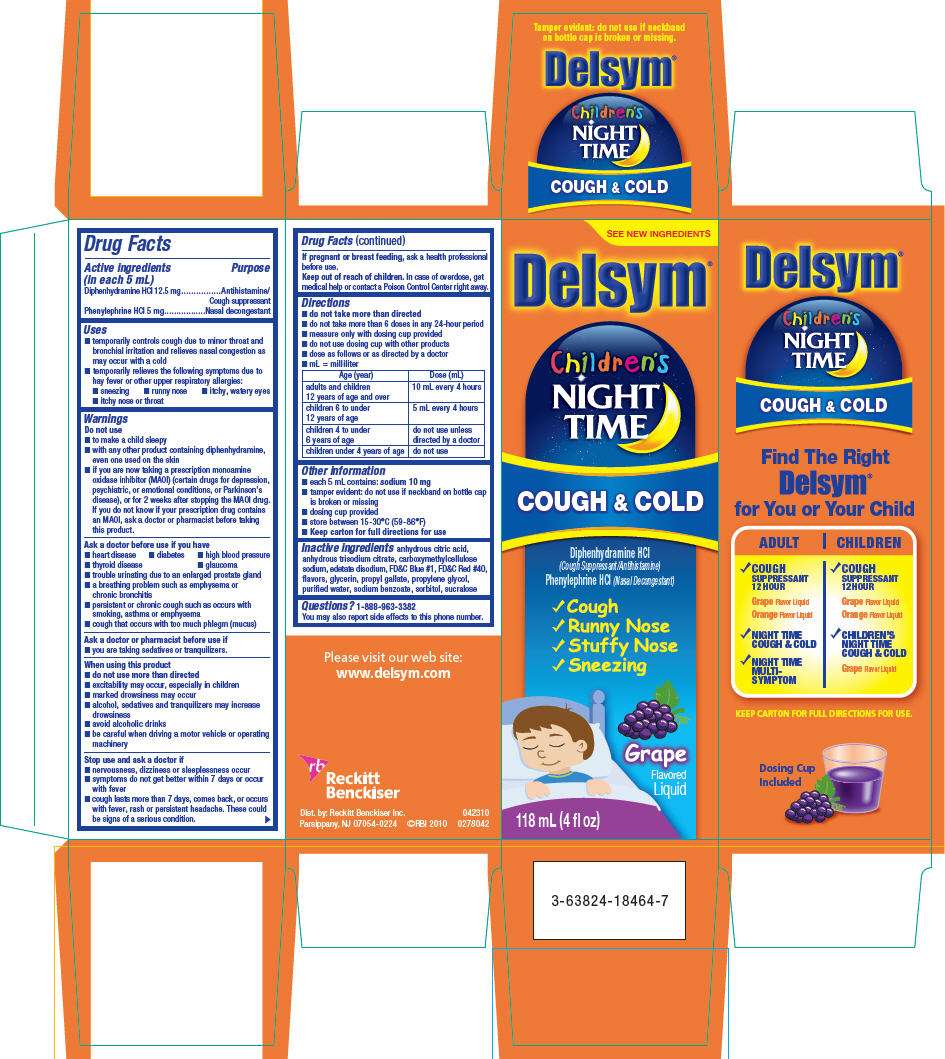
Drug Facts
Active ingredient
Purpose
| Active ingredients (in each 5 mL) | Purpose |
|---|---|
| Diphenhydramine HCl 12.5 mg | Antihistamine/Cough suppressant |
| Phenylephrine HCl 5 mg | Nasal decongestant |
Delsym Uses
- temporarily controls cough due to minor throat and bronchial irritation and relieves nasal congestion as may occur with a cold
- temporarily relieves the following symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- runny nose
- itchy, watery eyes
- itchy nose or throat
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on the skin
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- diabetes
- high blood pressure
- thyroid disease
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- persistent or chronic cough such as occurs with smoking, asthma or emphysema
- cough that occurs with too much phlegm (mucus)
Ask a doctor or pharmacist before use if
- you are taking sedatives or tranquilizers.
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness or sleeplessness occur
- symptoms do not get better within 7 days or occur with fever
- cough lasts more than 7 days, comes back, or occurs with fever, rash or persistent headache. These could be signs of a serious condition.
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than directed
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- dose as follows or as directed by a doctor
- mL = milliliter
| Age (year) | Dose (mL) |
|---|---|
| adults and children 12 years of age and over | 10 mL every 4 hours |
| children 6 to under 12 years of age | 5 mL every 4 hours |
| children 4 to under 6 years of age | do not use unless directed by a doctor |
| children under 4 years of age | do not use |
Delsym Other information
- each 5 mL contains: sodium 10 mg
- tamper evident: do not use if neckband on bottle cap is broken or missing
- dosing cup provided
- store between 15-30°C (59-86°F)
- Keep carton for full directions for use
Inactive ingredients
anhydrous citric acid, anhydrous trisodium citrate, carboxymethylcellulose sodium, edetate disodium, FD&C Blue #1, FD&C Red #40, flavors, glycerin, propyl gallate, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose
Questions?
1-888-963-3382
You may also report side effects to this phone number.
Dist. by: Reckitt Benckiser Inc.
Parsippany, NJ 07054-0224
PRINCIPAL DISPLAY PANEL - 118 mL Carton
SEE NEW INGREDIENTS
Delsym ®
Children's
NIGHT
TIME
COUGH & COLD
Diphenhydramine HCl
(Cough Suppressant/Antihistamine)
Phenylephrine HCl (Nasal Decongestant)
-
✓ Cough -
✓ Runny Nose -
✓ Stuffy Nose -
✓ Sneezing
Grape
Flavored
Liquid
118 mL (4 fl oz)
DelsymDiphenhydramine Hydrochloride and Phenylephrine Hydrochloride SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||