DELFLEX
Fresenius Medical Care North America
DELFLEX Dextrose Peritoneal Dialysis SolutionsFor Intraperitoneal Administration Only
FULL PRESCRIBING INFORMATION
The DELFLEX peritoneal dialysis solutions, standard, low magnesium and low magnesium/low calcium, are sterile, non-pyrogenic formulations of Dextrose and Electrolytes in Water for Injection, USP, for use in peritoneal dialysis. These solutions do not contain antimicrobial agents or additional buffers. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
Each 100 mL of standard dialysis solution contains 1.5, 2.5, or 4.25 g Dextrose, Hydrous, USP, 567 mg Sodium Chloride, USP, 392 mg Sodium Lactate, 25.7 mg Calcium Chloride, USP, 15.2 mg Magnesium Chloride, USP, q. s. Water for Injection, USP, Hydrochloric Acid or Sodium Hydroxide may be added for pH adjustment; pH is 5.5 (5.0 – 6.0).
DELFLEX WITH DEXTROSE Peritoneal Dialysis Solutions
| The total osmolarities shown in the above table are calculated theoretically. | |||||||||
| With 1.5% Dextrose |
With 2.5% Dextrose |
With 4.25% Dextrose |
|||||||
| Dextrose, H2O | 15 g/L | 25 g/L | 42.5 g/L | ||||||
| Sodium | 132 mEq/L | 132 mEq/L | 132 mEq/L | ||||||
| Calcium | 3.5 mEq/L | 3.5 mEq/L | 3.5 mEq/L | ||||||
| Magnesium | 1.5 mEq/L | 1.5 mEq/L | 1.5 mEq/L | ||||||
| Chloride | 102 mEq/L | 102 mEq/L | 102 mEq/L | ||||||
| Lactate | 35 mEq/L | 35 mEq/L | 35 mEq/L | ||||||
| Total Osmolarity | 347 mOsmol/L | 398 mOsmol/L | 486 mOsmol/L | ||||||
| PH (5.0 – 6.0) | 5.5 | 5.5 | 5.5 | ||||||
Each 100 mL of low magnesium dialysis solution contains 1.5, 2.5, or 4.25 g Dextrose, Hydrous, USP, 538 mg Sodium Chloride, USP, 448 mg Sodium Lactate, 25.7 mg Calcium Chloride, USP, 5.08 mg Magnesium Chloride, USP, q. s. Water for Injection, USP, Hydrochloric Acid or Sodium Hydroxide may be added for pH adjustment; pH is 5.5 (5.0 – 6.0).
DELFLEX LOW MAGNESIUM WITH DEXTROSE Peritoneal Dialysis Solutions
| The total osmolarities shown in the above table are calculated theoretically. | |||||||||
| With 1.5% Dextrose |
With 2.5% Dextrose |
With 4.25% Dextrose |
|||||||
| Dextrose, H2O | 15 g/L | 25 g/L | 42.5 g/L | ||||||
| Sodium | 132 mEq/L | 132 mEq/L | 132 mEq/L | ||||||
| Calcium | 3.5 mEq/L | 3.5 mEq/L | 3.5 mEq/L | ||||||
| Magnesium | 0.5 mEq/L | 0.5 mEq/L | 0.5 mEq/L | ||||||
| Chloride | 96 mEq/L | 96 mEq/L | 96 mEq/L | ||||||
| Lactate | 40 mEq/L | 40 mEq/L | 40 mEq/L | ||||||
| Total Osmolarity | 346 mOsmol/L | 396 mOsmol/L | 485 mOsmol/L | ||||||
| PH (5.0 – 6.0) | 5.5 | 5.5 | 5.5 | ||||||
Each 100 mL of low magnesium, low calcium dialysis solution contains 1.5, 2.5, or 4.25 g Dextrose, Hydrous, USP, 538 mg Sodium Chloride, USP, 448 mg Sodium Lactate, 18.4 mg Calcium Chloride, USP, 5.08 mg Magnesium Chloride, USP, q. s. Water for Injection, USP, Hydrochloric Acid or Sodium Hydroxide may be added for pH adjustment; pH is 5.5 (5.0 – 6.0).
DELFLEX LOW MAGNESIUN, LOW CALCIUM WITH DEXTROSE Peritoneal Dialysis Solutions
| The total osmolarities shown in the above table are calculated theoretically. | |||||||||
| With 1.5% Dextrose |
With 2.5% Dextrose |
With 4.25% Dextrose |
|||||||
| Dextrose, H2O | 15 g/L | 25 g/L | 42.5 g/L | ||||||
| Sodium | 132 mEq/L | 132 mEq/L | 132 mEq/L | ||||||
| Calcium | 2.5 mEq/L | 2.5 mEq/L | 2.5 mEq/L | ||||||
| Magnesium | 0.5 mEq/L | 0.5 mEq/L | 0.5 mEq/L | ||||||
| Chloride | 95 mEq/L | 95 mEq/L | 95 mEq/L | ||||||
| Lactate | 40 mEq/L | 40 mEq/L | 40 mEq/L | ||||||
| Total Osmolarity | 344 mOsmol/L | 394 mOsmol/L | 483 mOsmol/L | ||||||
| PH (5.0 – 6.0) | 5.5 | 5.5 | 5.5 | ||||||
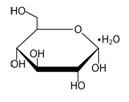
Dextrose USP, is a chemically designated D-glucose monohydrate (C6H12O6•H2O) a hexose sugar freely soluble in water. It has the following structural formula:
Calcium Chloride USP, a chemically designated calcium chloride dihydrate (CaCl2•2H2O) white fragments or granules freely soluble in water.
Magnesium Chloride USP, is chemically designated magnesium chloride hexahydrate (MgCl2•6H2O) colorless flakes or crystals very soluble in water.
Sodium Lactate Solution, USP, is chemically designated CH3CH(OH)COONa, a 60% aqueous solution miscible in water.
Sodium Chloride, USP, is chemically designated NaCl, a white, crystalline compound freely soluble in water.
Water for Injection, USP, is chemically designated H2O.
Since the flexible inner bag is compounded from a specific polyvinyl chloride, water may permeate from the inner bag into the outerwrap in quantities insufficient to affect the solution significantly. Solutions in contact with the plastic inner bag can leach out certain of its chemical components in very small amounts; however, the safety of the plastic formulation is supported by biological tests for plastic containers.
Peritoneal dialysis is the process of filtering excess water and toxins from the bloodstream through a semi-permeable membrane. This process does not cure the disease, but prevents progression of symptoms. Dialysis for chronic kidney failure is essential to maintain life, unless the patient receives a kidney transplant. A peritoneal dialysis procedure utilizes the peritoneum (lining of the abdomen) as the semi-permeable membrane. The procedure is conducted by instilling peritoneal dialysis solution through a catheter in the abdomen into the peritoneal cavity. Since the peritoneum is heavily supplied with blood vessels, the contact of the solution with the peritoneum causes excess water and toxins in the bloodstream to be drawn across the membrane into the solution. This osmosis and diffusion occurs between the plasma of the patient and the peritoneal dialysis solution. After a period of time called "dwell time," the solution is then drained from the patient.
This solution does not contain potassium. In situations in which there is a normal serum potassium level or hypokalemia, the addition of potassium chloride (up to a concentration of 4 mEq/L) may be indicated to prevent severe hypokalemia. Addition of potassium chloride should be made after careful evaluation of serum and total body potassium and only under the direction of a physician.
Clinical studies have demonstrated that the use of low magnesium solutions resulted in significant increases in serum CO2 and decreases in serum magnesium levels. The decrease in magnesium levels did not cause clinically significant hypomagnesemia.
DELFLEX® peritoneal dialysis solutions are indicated in the treatment of chronic renal failure patients being maintained on continuous ambulatory peritoneal dialysis when nondialytic medical therapy is judged to be inadequate.
None known.
Not for Intravenous Injection.
Use Aseptic Technique.
Peritoneal dialysis should be done with great care, in patients with a number of conditions, including disruption of the peritoneal membrane or diaphragm by surgery or trauma, extensive adhesions, bowel distention, undiagnosed abdominal disease, abdominal wall infection, hernias or burns, fecal fistula or colostomy, tense ascites, obesity, large polycystic kidneys, recent aortic graft replacement, lactic acidosis and severe pulmonary disease. When assessing peritoneal dialysis as the mode of therapy in such extreme situations, the benefits to the patient must be weighed against the possible complications.
Solutions containing lactate ion should be used with great care in patients with metabolic or respiratory alkalosis. Lactate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
An accurate fluid balance record must be kept and the weight of the patient carefully monitored to avoid over or under hydration with severe consequences, including congestive heart failure, volume depletion and shock.
Excessive use of DELFLEX peritoneal dialysis solution with 4.25% dextrose during a peritoneal dialysis treatment can result in significant removal of water from the patient.
Stable patients undergoing maintenance peritoneal dialysis should have routine periodic evaluation of blood chemistries and hematologic factors, as well as other indicators of patient status.
After removing the outer wrap, check for minute leaks by squeezing the container firmly. If leaks are found, discard the solution because the sterility may be impaired.
Serum calcium levels in patients using low calcium concentrations should be monitored and if found to be low, the peritoneal solution in use should be altered to one with a higher calcium concentration.
General:
DELFLEX® peritoneal dialysis solution should not be administered unless it is clear, all seals are intact, and there is no evidence of leaking.
Care should be taken to see that the catheter is inserted completely, since leakage around the catheter, if not controlled, can create edema from subcutaneous infiltration of the dialysis solution. This will also create an inaccurate fluid balance measurement.
Chronic patients that have been stabilized on peritoneal dialysis therapy should have routine evaluation of electrolyte blood chemistries and hematologic factors measured in order to determine the patient's ongoing condition.
DELFLEX PERITONEAL DIALYSIS SOLUTIONS DO NOT INCLUDE POTASSIUM. POTASSIUM CHLORIDE SHOULD ONLY BE ADDED UNDER THE DIRECTION OF A PHYSICIAN AFTER CAREFUL EVALUATION OF BOTH SERUM AND TOTAL BODY POTASSIUM.
Check the inner bag for leaks by gently squeezing the bag before removing the outer wrap. If after applying pressure on the bag, leaks are found, do not use this solution since the sterility of the bag may be compromised.
The outer wrap must be removed immediately before use and is provided with a “Tear Open” feature to make removal easy. See detailed instructions in this insert's last page.
Aseptic technique must be used throughout the procedure and at its termination in order to reduce the possibility of infection.
Significant loss of protein, amino acids and water soluble vitamins may occur during peritoneal dialysis. Replacement therapy should be provided as necessary.
Laboratory Tests:
Serum electrolytes, magnesium, bicarbonate levels and fluid balance should be periodically monitored.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long term animal studies with DELFLEX peritoneal dialysis solutions have not been performed to evaluate the carcinogenic potential, mutagenic potential or effect on fertility.
Pregnancy: Teratology Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with DELFLEX peritoneal dialysis solutions. It is also not known whether DELFLEX peritoneal dialysis solutions can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. DELFLEX peritoneal dialysis solutions should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Caution should be exercised when DELFLEX peritoneal dialysis solutions are administered to a nursing woman.
Pediatric Use:
Safety and effectiveness in pediatric patients have not been established.
Adverse reactions occurring with administration of peritoneal dialysis include mechanical and solution related problems as well as the results of contamination of equipment or improper technique in catheter placement. Abdominal pain, bleeding, peritonitis, subcutaneous infection around a peritoneal catheter, catheter blockage, difficulty in fluid removal, and ileus are among the complications of the procedure. Solution related adverse reactions may include peritonitis, catheter site infection, electrolyte and fluid imbalances, hypovolemia, hypervolemia, hypertension, hypotension, disequilibrium syndrome and muscle cramping.
If an adverse reaction does occur, institute appropriate therapeutic procedures according to the patient's needs and conditions, and save the remainder of the fluid in the bag for evaluation if deemed necessary.
DELFLEX® peritoneal dialysis solutions are provided for intraperitoneal administration only. The mode of therapy, frequency of treatment, formulation, exchange volume, duration of dwell, and length of dialysis should be selected by the physician responsible for the treatment of the individual patient.
To avoid the risk of severe dehydration or hypovolemia and to minimize the loss of protein, it is advisable to select the peritoneal dialysis solution with lowest level of osmolarity consistent with the fluid removal requirements for that exchange.
To Open
DELFLEX peritoneal dialysis solution flexible bags are supplied in an overwrap pouch. This outer wrap must be opened and removed immediately before use of the product and is provided with a “Tear-Open” feature to make opening easy.
Check the solution to assure that it is clear. Hold the bag up to a light source and visually inspect for particulate matter and discoloration prior to administration. Color may vary from clear to slightly yellow but does not affect efficacy and may be used. Some opacity may be observed in the plastic of the bag and/or tubing and is due to moisture absorption during the sterilization process. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Connect (Aseptic Technique is Required)
DELFLEX peritoneal dialysis solution utilize a Safe-Lock® Connection System. This unique system consists of two Safe-Lock connectors, one located on the administration port of the bag, and the mating connector is located on the fluid delivery set. The Safe-Lock connectors were designed to prevent touch contamination of the internal connection components.
To connect the bag to the fluid delivery set, unscrew the protective caps of the bag connector and fluid delivery set connector. Secure these two connectors with a twisting motion to lock in place, so that the fluid delivery set connector is seated over the bag connector O-ring to assure a firm and tight fit.
Once the fluid delivery set is secured, to initiate solution flow, break the cone of the bag connector by placing the thumb firmly on the tube over the cone and pressing towards the outer wall of the tube and away from the bag. Once the cone is broken, a white retaining guide maintains the cone at a specific distance from the connector so it will not impede the flow of solution through the Safe-Lock connector.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Additives may be incompatible. Do not store solutions containing additives.
DELFLEX peritoneal dialysis solutions are delivered in single-dose flexible bags.All DELFLEX peritoneal dialysis solutions have overfills declared on the container label.The flexible containers have the capacity for drainage in excess of their stated fill volume for ultrafiltration from the patient.
DELFLEX peritoneal dialysis solutions are available in the following sizes and formulations:
| 1.5% Dextrose | |||||||
| Ca, mEq/L | 3.5 (Standard) | 3.5 (Standard) | 2.5 (Low) | ||||
| Mg, mEq/L | 1.5 (Standard) | 0.5 (Low) | 0.5 (Low) | ||||
| 1 liter | X | X | X | ||||
| 1.5 liter/2L bag | X | X | X | ||||
| 2 liter | X | X | X | ||||
| 2 liter/3L bag | X | X | X | ||||
| 2.5 liter/3L bag | X | X | X | ||||
| 3 liter | X | X | X | ||||
| 5 liter | X | X | X | ||||
| 2.5% Dextrose | |||||||
| Ca, mEq/L | 3.5 (Standard) | 3.5 (Standard) | 2.5 (Low) | ||||
| Mg, mEq/L | 1.5 (Standard) | 0.5 (Low) | 0.5 (Low) | ||||
| 1 liter | X | X | X | ||||
| 1.5 liter/2L bag | X | X | X | ||||
| 2 liter | X | X | |||||
| 2 liter/3L bag | X | X | X | ||||
| 2.5 liter/3L bag | X | X | X | ||||
| 3 liter | X | X | X | ||||
| 5 liter | X | X | X | ||||
| 4.25% Dextrose | |||||||
| Ca, mEq/L | 3.5 (Standard) | 3.5 (Standard) | 2.5 (Low) | ||||
| Mg, mEq/L | 1.5 (Standard) | 0.5 (Low) | 0.5 (Low) | ||||
| 1 liter | X | X | X | ||||
| 1.5 liter/2L bag | X | X | X | ||||
| 2 liter | X | X | |||||
| 2 liter/3L bag | X | X | X | ||||
| 2.5 liter/3L bag | X | X | X | ||||
| 3 liter | X | X | X | ||||
| 5 liter | X | X | X | ||||
STORAGE CONDITIONS
STORE AT ROOM TEMPERATURE (25°C). Protect from freezing and extreme heat.
Fresenius Medical Care North America
920 Winter Street
Waltham, MA 02451
1-800-323-5188
89-905-61 Rev 07/11
Principal Display Panel - NDC 49230-206-50

Principal Display Panel - NDC 49230-209-50

Principal Display Panel - NDC 49230-212-50

DELFLEXDextrose Monohydrate, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride Solution
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DELFLEXDextrose Monohydrate, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride Solution
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DELFLEXDextrose Monohydrate, Sodium Chloride, Sodium Lactate, Calcium Chloride, Magnesium Chloride Solution
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||