DEFENDER Antiseptic Foam Hand
Scientific Molecular Technologies
DEFENDER Antiseptic Foam Hand
FULL PRESCRIBING INFORMATION: CONTENTS*
- Drug Facts
- Active Ingredients
- Purpose
- DEFENDER Antiseptic Foam Hand Uses
- Warnings
- Directions
- Inactive Ingredients
- Product Label
FULL PRESCRIBING INFORMATION
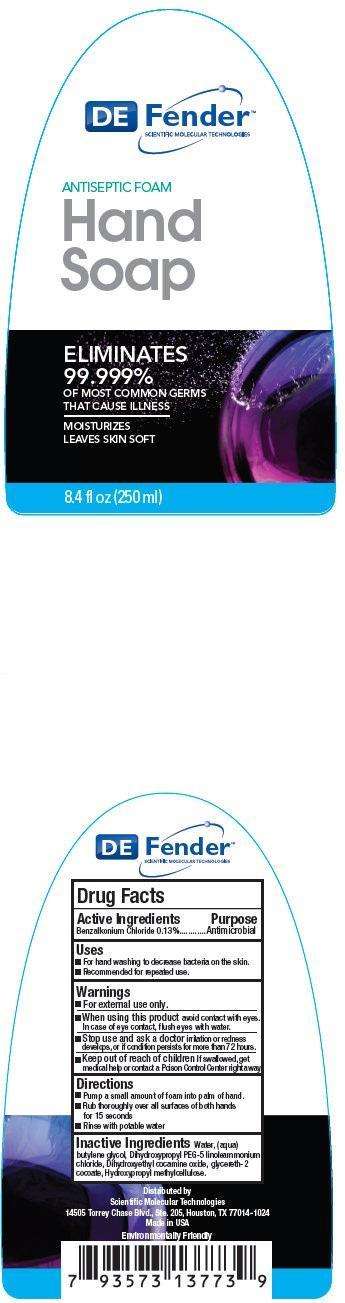
Drug Facts
Active Ingredients
Benzalkonium Chloride 0.13%
Purpose
Antimicrobial
DEFENDER Antiseptic Foam Hand Uses
- For hand washing to decrease bacteria on the skin.
- Recommended for repeated use.
Warnings
-
For external use only.
When using this product
avoid contact with eyes. In case of eye contact, flush eyes with water.
Stop use and ask a doctor
irritation or redness develops. If condition persists for more than 72 hours.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Pump a small amount of foam into palm of hand.
- Run thoroughly over all surfaces of both hands for 15 seconds
- Rinse with potable water.
Inactive Ingredients
Water, (aqua), butylene glycol, Dihydroxypropyl PEG-5 linoleammonium chloride, Dihydroxyethyl cocamine oxide, glycereth-2 cocoate, Hydroxypropyl methylcellulose.
DE Fender SCIENTIFIC MOLECULAR TECHNOLOGIES ANTISEPTIC FOAM Hand Soap ELIMINATES 99.999% OF MOST COMMON GERMS THAT CAUSES ILLNESS NO RINSE MOISTURIZES LEAVES SKIN SOFT 8.4 fl oz (250 ml)
Product Label
DEFENDER Antiseptic Foam HandBENZALKONIUM CHLORIDE SOAP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||