Dectomax
Pfizer Animal Health
Pfizer Inc.
DECTOMAX (doramectin)
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRODUCT DECTOMAX DESCRIPTION
- PRODUCT CHARACTERISTICS
- PRODUCT INDICATIONS
- DOSAGE
- ADMINISTRATION
- WARNINGS
- RESIDUE WARNINGS
- PRECAUTIONS
- ENVIRONMENTAL SAFETY
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 100 mL Vial Label
FULL PRESCRIBING INFORMATION
Antiparasitic
1% injectable solution for cattle and swine
10 mg/mL
PRODUCT DESCRIPTION
Dectomax injectable solution is a ready-to-use, colorless to pale yellow, sterile solution containing 1% w/v doramectin (10 mg/mL). In cattle, Dectomax is formulated to deliver the recommended dosage (200 mcg/kg of body weight) when given by subcutaneous (SC) or intramuscular (IM) injection at the rate of 1 mL/110 lb of body weight. In swine, Dectomax is formulated to deliver the recommended dosage (300 mcg/kg of body weight) when given by IM injection at the rate of 1 mL/75 lb of body weight.
PRODUCT CHARACTERISTICS
Dectomax injectable solution is a highly active, broad-spectrum parasiticide for parenteral administration to cattle and swine. It contains doramectin, a novel fermentation-derived macrocyclic lactone discovered by Pfizer Inc. Doramectin is isolated from fermentations of selected strains derived from the soil organism Streptomyces avermitilis.
A primary mode of action of macrocyclic lactones is to modulate chloride ion channel activity in the nervous system of nematodes and arthropods. Macrocyclic lactones bind to receptors that increase membrane permeability to chloride ions. This inhibits the electrical activity of nerve cells in nematodes and muscle cells in arthropods and causes paralysis and death of the parasites. In mammals, the neuronal receptors to which macrocyclic lactones bind are localized within the central nervous system (CNS), a site reached by only negligible concentrations of doramectin.
One dose of Dectomax injectable solution effectively treats and controls a wide range of roundworm and arthropod parasites that impair the health and productivity of cattle and swine.
Studies have demonstrated the safety margin of Dectomax injection in cattle and swine. In USA trials, no toxic signs were seen in cattle given up to 25 times the recommended dose, or in swine given up to 10 times the recommended dose. Studies also demonstrated safety in neonatal calves and piglets treated with up to 3 times the recommended dose. In males (bulls and boars) and females (cows and sows during folliculogenesis, implantation, organogenesis, and through gestation), a dose 3 times the recommended dose had no effect on breeding performance.
PRODUCT INDICATIONS
Cattle
Dectomax injectable solution is indicated for the treatment and control of the following harmful species of gastrointestinal roundworms, lungworms, eyeworms, grubs (see PRECAUTIONS), sucking lice (see PRECAUTIONS), and mange mites. Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
| Gastrointestinal Roundworms (adults and fourth stage larvae) | Lungworms (adults and fourth stage larvae) |
| Ostertagia ostertagi (including inhibited larvae) | Dictyocaulus viviparus |
| O. lyrata | Eyeworms (adults) |
| Haemonchus placei | Thelazia spp. |
| Trichostrongylus axei | Grubs (parasitic stages) |
| T. colubriformis | Hypoderma bovis |
T. longispicularis |
H. lineatum |
| Cooperia oncophora | Sucking Lice |
C. pectinata |
Haematopinus eurysternus |
| C. punctata | Linognathus vituli |
| C. surnabada (syn. mcmasteri) | Solenopotes capillatus |
Bunostomum phlebotomum |
Mange Mites |
Strongyloides papillosus |
Psoroptes bovis |
| Oesophagostomum radiatum | Sarcoptes scabiei |
Trichuris spp. |
Dectomax injectable solution has been proved to effectively control infections and to protect cattle from reinfection with Cooperia oncophora and Haemonchus placei for 14 days, Ostertagia ostertagi for 21 days, and C. punctata, Oesophagostomum radiatum, and Dictyocaulus viviparus for 28 days after treatment.
Swine
Dectomax injectable solution is indicated for the treatment and control of the following species of gastrointestinal roundworms, lungworms, kidney worms, sucking lice (see PRECAUTIONS), and mange mites. Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
| Gastrointestinal Roundworms (adults and fourth stage larvae) | Lungworms (adults) |
| Ascaris suum | Metastrongylus spp. |
| Oesophagostomum dentatum | Kidney Worms (adults) |
Oesophagostomum quadrispinulatum |
Stephanurus dentatus |
Strongyloides ransomi |
Mange Mites (adults and immature stages) |
Hyostrongylus rubidus |
Sarcoptes scabiei var. suis |
| Sucking Lice (adults and immature stages) | |
| Haematopinus suis |
DOSAGE
Cattle
Administer Dectomax injectable solution at the recommended dosage of 200 mcg doramectin per kg (91 mcg/lb) of body weight. Each mL contains 10 mg of doramectin, sufficient to treat 110 lb (50 kg) of body weight.
| Body Weight (lb) | Dose (mL) |
|---|---|
| 110 | 1 |
| 220 | 2 |
| 330 | 3 |
| 440 | 4 |
| 550 | 5 |
| 660 | 6 |
| 770 | 7 |
| 880 | 8 |
| 990 | 9 |
| 1,100 | 10 |
Swine
Administer Dectomax injectable solution at the recommended dosage of 300 mcg doramectin per kg (136 mcg/lb) of body weight. Each mL contains 10 mg of doramectin, sufficient to treat 75 lb (34 kg) of body weight.
| Body Weight (lb) | Dose (mL) |
|---|---|
| 15 | 0.2 |
| 30 | 0.4 |
| 45 | 0.6 |
| 60 | 0.8 |
| 75 | 1.0 |
| 150 | 2.0 |
| 225 | 3.0 |
| 300 | 4.0 |
| 375 | 5.0 |
| 450 | 6.0 |
RECOMMENDED TREATMENT PROGRAM FOR SWINE
To effectively initiate control of mange and sucking lice in swine, it is important to treat all animals in the herd. After initial treatment, use Dectomax regularly as follows:
Breeding Animals:
Sows: Treat 7–14 days prior to farrowing to minimize exposure of piglets to mites and sucking lice.
Gilts: Treat 7–14 days prior to breeding. Treat 7–14 days prior to farrowing.
Boars: Treat a minimum of 2 times per year.
Feeder Pigs: Treat any new feeder pigs upon arrival at farm or before placement in clean quarters.
Weaners, Growers, Finishers: Weaners and grow-out/finisher pigs should be treated before placement in clean quarters.
For effective mange elimination, care must be taken to prevent reinfestation from exposure to untreated animals or contaminated facilities.
ADMINISTRATION
Dry, sterile equipment and aseptic procedures should be used when withdrawing and administering Dectomax. For multiple treatments either automatic injection equipment or an aspirating needle should be used.
Cattle
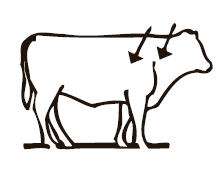
Administer Dectomax injectable solution by SC or IM route. Injections should be made using a 16 gauge needle for adult cattle or an 18 gauge needle for young animals. Needles 1/2–3/4" in length are suggested for SC administration. A 1-1/2" needle is suggested for IM administration. SC injections should be administered under the loose skin in front of or behind the shoulder. IM injections should be administered into the muscular region of the neck. Beef Quality Assurance guidelines recommend SC administration as the preferred route.
Swine
Administer Dectomax injectable solution by the IM route. Inject in the neck region using an 18 gauge × 1" needle for young animals; a 16 gauge × 1-1/2" needle for sows and boars. To accurately meter doses administered to piglets, use of a tuberculin syringe and 20 gauge × 1" needle is recommended.

WARNINGS
Not for human use. Keep out of reach of children. The material safety data sheet (MSDS) contains more detailed occupational safety information. To report adverse effects in users, to obtain more information, or to obtain an MSDS, call 1-800-366-5288.
RESIDUE WARNINGS
Cattle
Do not slaughter for human consumption within 35 days of treatment. Not for use in female dairy cattle 20 months of age or older. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal.
Swine
Do not slaughter for human consumption within 24 days of treatment.
PRECAUTIONS
Dectomax has been developed specifically for use in cattle and swine only. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
For SC injection in cattle only. For IM injection in swine and cattle. This product is approved for the treatment and control of sucking lice. For treatment of biting lice in cattle, use of Dectomax Pour-On is recommended.
Dectomax is highly effective against all stages of cattle grubs. However, proper timing of treatment is important. For most effective results, cattle should be treated as soon as possible after the end of the heel fly (warble) season.
Destruction of Hypoderma larvae (cattle grubs) at the period when these grubs are in vital areas may cause undesirable host-parasite reactions including the possibility of fatalities. Killing H. lineatum when it is in the tissue surrounding the gullet may cause bloat; killing H. bovis when it is in the vertebral canal may cause staggering or paralysis. These reactions are not specific to treatment with Dectomax, but can occur with any successful treatment of grubs. Cattle should be treated either before or after these stages of grub development. Consult your veterinarian concerning the proper time for treatment.
Cattle treated with Dectomax after the end of the heel fly season may be re-treated with Dectomax during the winter for internal parasites, mange mites, or sucking lice, without danger of grub-related reactions. A planned parasite control program is recommended.
ENVIRONMENTAL SAFETY
Studies indicate that when doramectin comes in contact with the soil, it readily and tightly binds to the soil and becomes inactive over time. Free doramectin may adversely affect fish and certain aquatic organisms. Do not permit water runoff from feedlots to enter streams or ponds. Do not contaminate water by direct application or by the improper disposal of drug containers. Dispose of containers in an approved landfill or by incineration.
As with other avermectins, doramectin is excreted in the dung of treated animals and can inhibit the reproduction and growth of pest and beneficial insects that use dung as a source of food and for reproduction. The magnitude and duration of such effects are species and life-cycle specific. When used according to label directions, the product is not expected to have an adverse impact on populations of dung-dependent insects.
Store Below 30°C (86°F)
HOW SUPPLIED
Dectomax is available in 100-mL, 200-mL, and 500-mL multi-dose, rubber-capped glass vials.
U.S. Patent No. 5,089,480
NADA #141-061, Approved by FDA
Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
Not for human use
Restricted Drug (CA) Use only as directed.
Licensed in the Ministry of Agriculture under no 4.055/92, on 8/14/92
Distributed by:
Pfizer Animal Health
Div. of Pfizer Inc
NY, NY 10017

79-5195-00-9
July 2005
036253
PRINCIPAL DISPLAY PANEL - 100 mL Vial Label
DECTOMAX®
(doramectin)
Antiparasitic
1% injectable solution
for cattle and swine
10 mg/mL
Net Contents: 100 mL
NADA #141-061, Approved by FDA
036253
Pfizer

Dectomaxdoramectin INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||