Dawn
Procter & Gamble Manufacturing Company
Dawn Ultra Concentrated Orange AB
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
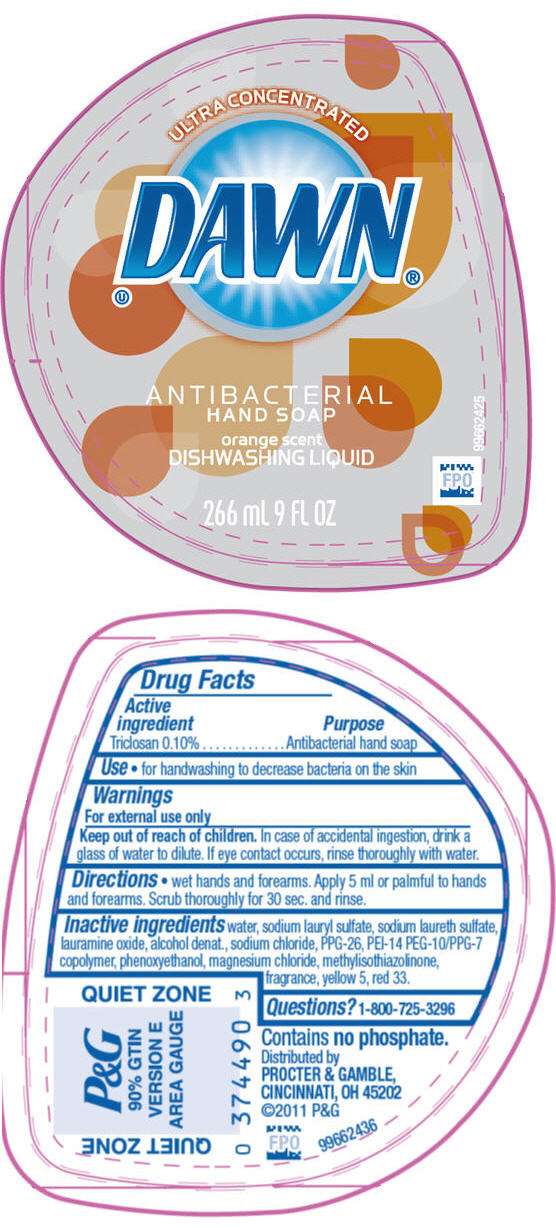
- PRINCIPAL DISPLAY PANEL - 266 mL Bottle Label
FULL PRESCRIBING INFORMATION
DRUG FACTS
Active Ingredient
Triclosan 0.10%
Purpose
Antibacterial hand soap
Use
- For handwashing to decrease bacteria on the skin
Warnings
For external use only
Keep out of reach of children.
In case of accidental ingestion, drink a glass of water to dilute. If eye contact occurs, rinse thoroughly with water.
Directions
- Wet hands and forearms. Apply 5 ml or palmful to hands and forearms. Scrub thoroughly for 30 sec. and rinse.
Inactive ingredients
water, sodium lauryl sulfate, sodium laureth sulfate, lauramine oxide, alcohol denat., sodium chloride, PPG-26, PEI-14 PEG-10/PPG-7 copolymer, phenoxyethanol, magnesium chloride, methylisothiazolinone, fragrance, yellow 5, red 33.
Questions?
1-800-725-3296
Distributed by PROCTER & GAMBLE, CINCINNATI, OH 45202.
PRINCIPAL DISPLAY PANEL - 266 mL Bottle Label
ULTRA CONCENTRATED
DAWN
®
Ⓤ
ANTIBACTERIAL
HAND SOAP
orange scent
DISHWASHING LIQUID
266 mL 9 FL OZ
FPO
99662425
DawnTriclosan SOAP
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||