DAILY MOISTURIZING
BACK LABEL - DRUG FACTS BOX
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- WARNING
- Purpose
- QUESTIONS/COMMENTS?
- DAILY MOISTURIZING Uses
- Directions
- Inactive Ingredients
- Package Front and Back Labels
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
DIMETHICONE 1.25%
WARNING
FOR EXTERNAL USE ONLY.
When Using This Product
AVOID CONTACT WITH EYES, IF CONTACT OCCURS, RINSE EYE THROUGHLY WITH WATER.
STOP USING THIS PRODUCT AND ASK A DOCTOR IF
CONDITION WORSENS OR SYMPTOMS PERSIST FOR MORE THAN SEVEN DAYS OR OCCUR AGAIN WITHIN A FEW DAYS.
KEEP OUT OF REACH OF CHILDREN
IN CASE OF ACCIDENTAL INGESTION, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Purpose
QUESTIONS/COMMENTS?
1 - 877 - 932 - 7948
DAILY MOISTURIZING Uses
Directions
Inactive Ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Sodium Chloride, DMDM Hydantoin, Methylparaben, Propylparaben
Package Front and Back Labels
18OZ Front and Back Labels: equaline18.jpg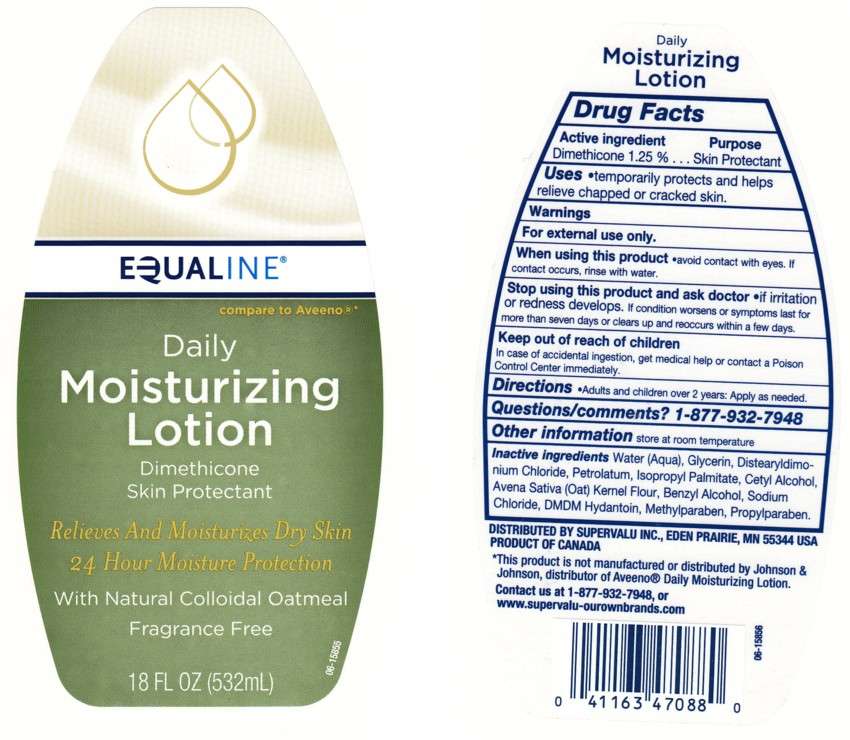
DAILY MOISTURIZINGDIMETHICONE LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||