Cyclobenzaprine Hydrochloride
Bryant Ranch Prepack
Bryant Ranch Prepack
CYCLOBENZAPRINE HYDROCHLORIDE TABLETS, USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- CYCLOBENZAPRINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CYCLOBENZAPRINE HYDROCHLORIDE INDICATIONS AND USAGE
- CYCLOBENZAPRINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CYCLOBENZAPRINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- CYCLOBENZAPRINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- Cyclobenzaprine 5mg Tablet
FULL PRESCRIBING INFORMATION
CYCLOBENZAPRINE HYDROCHLORIDE DESCRIPTION
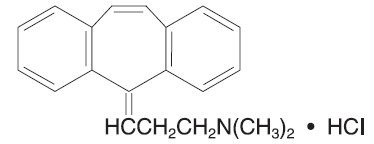
Cyclobenzaprine hydrochloride is a white, crystalline tricyclic amine salt with the empirical formula C H N • HCl and a molecular weight of 311.9. It has a melting point of 217°C, and a pK of 8.47 at 25°C. It is freely soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base separates. 20 21 a
Cyclobenzaprine HCl is designated chemically as 3-( -dibenzo[ ]cyclohepten-5-ylidene)- , -dimethyl-1-propanamine hydrochloride, and has the following structural formula: 5H a,d N N
Cyclobenzaprine Hydrochloride Tablets, USP are supplied as 5 mg and 10 mg tablets for oral administration.
Each tablet contains the following inactive ingredients: croscarmellose sodium, FD&C Yellow #6, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, and titanium dioxide; 5 mg tablets also contain FD&C Red #40 and 10 mg tablets contain D&C Yellow #10 and polysorbate.
CLINICAL PHARMACOLOGY
Cyclobenzaprine HCl relieves skeletal muscle spasm of local origin without interfering with muscle function. It is ineffective in muscle spasm due to central nervous system disease.
Cyclobenzaprine reduced or abolished skeletal muscle hyperactivity in several animal models. Animal studies indicate that cyclobenzaprine does not act at the neuromuscular junction or directly on skeletal muscle. Such studies show that cyclobenzaprine acts primarily within the central nervous system at brain stem as opposed to spinal cord levels, although its action on the latter may contribute to its overall skeletal muscle relaxant activity. Evidence suggests that the net effect of cyclobenzaprine is a reduction of tonic somatic motor activity, influencing both gamma (γ) and alpha (α) motor systems.
Pharmacological studies in animals showed a similarity between the effects of cyclobenzaprine and the structurally related tricyclic antidepressants, including reserpine antagonism, norepinephrine potentiation, potent peripheral and central anticholinergic effects, and sedation. Cyclobenzaprine caused slight to moderate increase in heart rate in animals.
Clinical Studies
Eight double-blind controlled clinical studies were performed in 642 patients comparing cyclobenzaprine hydrochloride 10 mg, diazepam, and placebo. Muscle spasm, local pain and tenderness, limitation of motion, and restriction in activities of daily living were evaluated. In three of these studies there was a significantly greater improvement with cyclobenzaprine than with diazepam, while in the other studies the improvement following both treatments was comparable.
Although the frequency and severity of adverse reactions observed in patients treated with cyclobenzaprine were comparable to those observed in patients treated with diazepam, dry mouth was observed more frequently in patients treated with cyclobenzaprine and dizziness more frequently in those treated with diazepam. The incidence of drowsiness, the most frequent adverse reaction, was similar with both drugs.
The efficacy of cyclobenzaprine hydrochloride tablets 5 mg was demonstrated in two seven-day, double-blind, controlled clinical trials enrolling 1405 patients. One study compared cyclobenzaprine hydrochloride tablets 5 and 10 mg t.i.d. to placebo; and a second study compared cyclobenzaprine hydrochloride tablets 5 and 2.5 mg t.i.d. to placebo. Primary endpoints for both trials were determined by patient-generated data and included global impression of change, medication helpfulness, and relief from starting backache. Each endpoint consisted of a score on a 5-point rating scale (from 0 or worst outcome to 4 or best outcome). Secondary endpoints included a physician's evaluation of the presence and extent of palpable muscle spasm.
Comparisons of cyclobenzaprine hydrochloride tablets 5 mg and placebo groups in both trials established the statistically significant superiority of the 5 mg dose for all three primary endpoints at day 8 and, in the study comparing 5 and 10 mg, at day 3 or 4 as well. A similar effect was observed with cyclobenzaprine hydrochloride tablets 10 mg (all endpoints). Physician-assessed secondary endpoints also showed that cyclobenzaprine hydrochloride tablets 5 mg was associated with a greater reduction in palpable muscle spasm than placebo.
Analysis of the data from controlled studies shows that cyclobenzaprine produces clinical improvement whether or not sedation occurs.
A post-marketing surveillance program was carried out in 7607 patients with acute musculoskeletal disorders, and included 297 patients treated with cyclobenzaprine hydrochloride tablets 10 mg for 30 days or longer. The overall effectiveness of cyclobenzaprine was similar to that observed in the double-blind controlled studies; the overall incidence of adverse effects was less (see ). ADVERSE REACTIONS
CYCLOBENZAPRINE HYDROCHLORIDE INDICATIONS AND USAGE
Cyclobenzaprine hydrochloride tablets are indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions.
Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, limitation of motion, and restriction in activities of daily living.
Cyclobenzaprine hydrochloride tablets should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
Cyclobenzaprine hydrochloride tablets have not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease, or in children with cerebral palsy.
CYCLOBENZAPRINE HYDROCHLORIDE CONTRAINDICATIONS
Hypersensitivity to any component of this product.
Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures, and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
Acute recovery phase of myocardial infarction, and patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
Hyperthyroidism.
WARNINGS
Cyclobenzaprine is closely related to the tricyclic antidepressants, e.g., amitriptyline and imipramine. In short term studies for indications other than muscle spasm associated with acute musculoskeletal conditions, and usually at doses somewhat greater than those recommended for skeletal muscle spasm, some of the more serious central nervous system reactions noted with the tricyclic antidepressants have occurred (see , below, and ). WARNINGS ADVERSE REACTIONS
Tricyclic antidepressants have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke.
Cyclobenzaprine may enhance the effects of alcohol, barbiturates, and other CNS depressants.
PRECAUTIONS
CYCLOBENZAPRINE HYDROCHLORIDE ADVERSE REACTIONS
Incidence of most common adverse reactions in the 2 double-blind , placebo-controlled 5 mg studies (incidence of > 3% on cyclobenzaprine hydrochloride tablets 5 mg): ‡
| Cyclobenzaprine Hydrochloride Tablets 5 mg N=464
|
Cyclobenzaprine Hydrochloride Tablets 10 mg N=249
|
Placebo N=469
|
|
| Drowsiness | 29% | 38% | 10% |
| Dry Mouth | 21% | 32% | 7% |
| Fatigue | 6% | 6% | 3% |
| Headache | 5% | 5% | 8% |
Adverse reactions which were reported in 1% to 3% of the patients were: abdominal pain, acid regurgitation, constipation, diarrhea, dizziness, nausea, irritability, mental acuity decreased, nervousness, upper respiratory infection, and pharyngitis.
The following list of adverse reactions is based on the experience in 473 patients treated with cyclobenzaprine hydrochloride tablets 10 mg in additional controlled clinical studies, 7607 patients in the post-marketing surveillance program, and reports received since the drug was marketed. The overall incidence of adverse reactions among patients in the surveillance program was less than the incidence in the controlled clinical studies.
The adverse reactions reported most frequently with cyclobenzaprine hydrochloride were drowsiness, dry mouth and dizziness. The incidence of these common adverse reactions was lower in the surveillance program than in the controlled clinical studies:
‡ Note: Cyclobenzaprine hydrochloride tablets 10 mg data are from one clinical trial. Cyclobenzaprine hydrochloride tablets 5 mg and placebo data are from two studies.
| Clinical Studies with Cyclobenzaprine Hydrochloride Tablets 10 mg
|
Surveillance Program with Cyclobenzaprine Hydrochloride Tablets 10 mg
|
|
| Drowsiness | 39% | 16% |
| Dry Mouth | 27% | 7% |
| Dizziness | 11% | 3% |
Among the less frequent adverse reactions, there was no appreciable difference in incidence in controlled clinical studies or in the surveillance program. Adverse reactions which were reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion.
The following adverse reactions have been reported in post-marketing experience or with an incidence of less than 1% of patients in clinical trials with the 10 mg tablet:
Syncope; malaise. Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension. Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice and cholestasis. Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash. Local weakness. Seizures, ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis, abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia. Sweating. Ageusia; tinnitus. Urinary frequency and/or retention.
Body as a Whole:
Cardiovascular:
Digestive:
Hypersensitivity:
Musculoskeletal:
Nervous System and Psychiatric:
Skin:
Special Senses:
Urogenital:
Causal Relationship Unknown
Other reactions, reported rarely for cyclobenzaprine hydrochloride under circumstances where a causal relationship could not be established or reported for other tricyclic drugs, are listed to serve as alerting information to physicians:
Chest pain; edema. Hypertension; myocardial infarction; heart block; stroke. Paralytic ileus; tongue discoloration; stomatitis; parotid swelling. Inappropriate ADH syndrome. Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia. Elevation and lowering of blood sugar levels; weight gain or loss. Myalgia. Decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell's palsy; alteration in EEG patterns; extrapyramidal symptoms. Dyspnea. Photosensitization; alopecia. Impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea.
Body as a Whole:
Cardiovascular:
Digestive:
Endocrine:
Hematic and Lymphatic:
Metabolic, Nutritional and Immune:
Musculoskeletal:
Nervous System and Psychiatric:
Respiratory:
Skin:
Urogenital:
DRUG ABUSE AND DEPENDENCE
Pharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when cyclobenzaprine hydrochloride is administered, even though they have not been reported to occur with this drug. Abrupt cessation of treatment after prolonged administration rarely may produce nausea, headache, and malaise. These are not indicative of addiction.
OVERDOSAGE
Although rare, deaths may occur from overdosage with cyclobenzaprine hydrochloride. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose. Signs and symptoms of toxicity may develop rapidly after cyclobenzaprine overdose; therefore, hospital monitoring is required as soon as possible. The acute oral LD of cyclobenzaprine hydrochloride is approximately 338 and 425 mg/kg in mice and rats, respectively. As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. 50
CYCLOBENZAPRINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
For most patients, the recommended dose of cyclobenzaprine hydrochloride tablets is 5 mg three times a day. Based on individual patient response, the dose may be increased to 10 mg three times a day. Use of cyclobenzaprine hydrochloride tablets for periods longer than two or three weeks is not recommended (see ). INDICATIONS AND USAGE
Less frequent dosing should be considered for hepatically impaired or elderly patients (see , and ). PRECAUTIONS, Impaired Hepatic Function Use in the Elderly
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Cyclobenzaprine 5mg Tablet

Cyclobenzaprine Hydrochloridecyclobenzaprine hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||