Cyclobenzaprine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- CYCLOBENZAPRINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CYCLOBENZAPRINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CYCLOBENZAPRINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
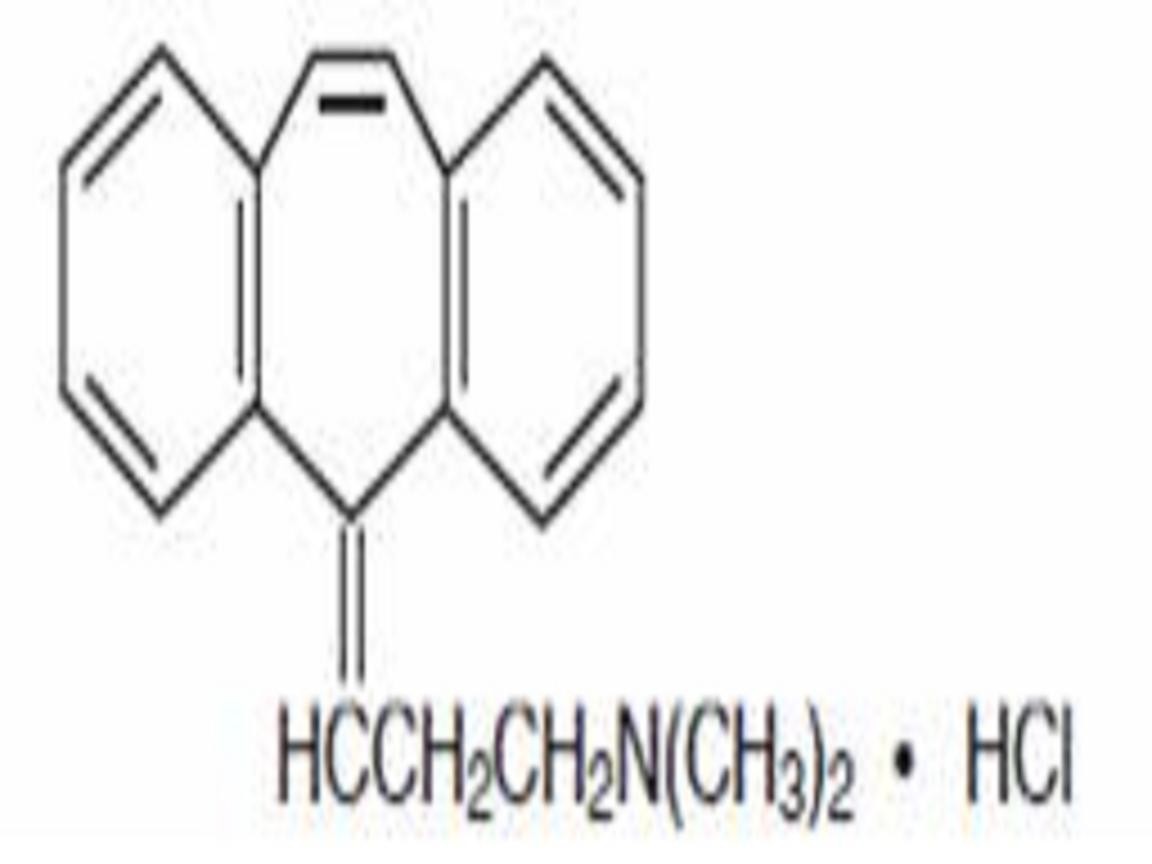
CYCLOBENZAPRINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PRECAUTIONS, Use in the ElderlyPRECAUTIONS, Impaired Hepatic Function
ADVERSE REACTIONS
INDICATIONS & USAGE
CYCLOBENZAPRINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
ADVERSE REACTIONSPRECAUTIONS
CLINICAL PHARMACOLOGY, Pharmacokinetics, Hepatic Impairment
CONTRAINDICATIONS
CLINICAL PHARMACOLOGY, Pharmacokinetics, Elderly
CYCLOBENZAPRINE HYDROCHLORIDE ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
DOSAGE & ADMINISTRATION
INDICATIONS AND USAGEPRECAUTIONS, Impaired Hepatic FunctionUse in the Elderly
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Cyclobenzaprine HydrochlorideCyclobenzaprine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!