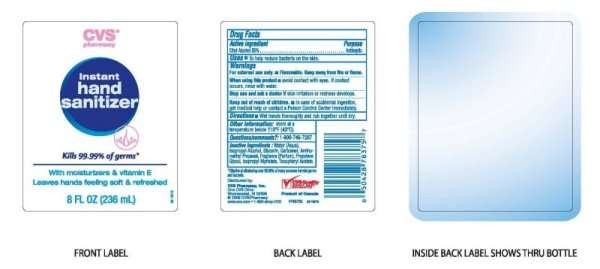
CVS Pharmacy Instant Hand Sanitizer With Moisturizers And Vitamin E
CVS Pharmacy
Cinogen Cosmetics Zhaoqing, LTD.
CVS Instant Hand Sanitizer With Moisturizers & Vitamin E
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient Purpose
Ethyl Alchohol 65 Percent Antiseptic
Purpose
Uses
To help reduce bacteria on the skin.
Warnings
For external use only.
Flammable. Keep away from fire or flame.
When using this product
Avoid contact with eyes.
If contact occurs, rinse with water.
Stop use and ask a doctor if skin irritation or redness develops.
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Directions
Wet hands thoroughly and rub together until dry.
Other information:
Store at a temperature below 110 degrees F (43 degrees C)
Questions Comments ? : 1-800-746-7287
Inactive ingredients: Water aqua, Isopropyl Alcohol, Glycerin, Carbomer, Aminomethyl Propanol, Fragrance Parfum,
Propylene Glycol, Isopropyl Myristate, Tocopheryl Acetate.
Effective at eliminating over 99.99 percent of many common harmful germs and bacteria.
CVS Pharmacy
Instant Hand Sanitizer
Kills 99.99 percent of germs
With Moisturizers And Vitamin E
Leaves hands feeling soft and refreshed
8 Fl OZ 236 ml

CVS Pharmacy Instant Hand Sanitizer With Moisturizers And Vitamin EALCOHOL GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||