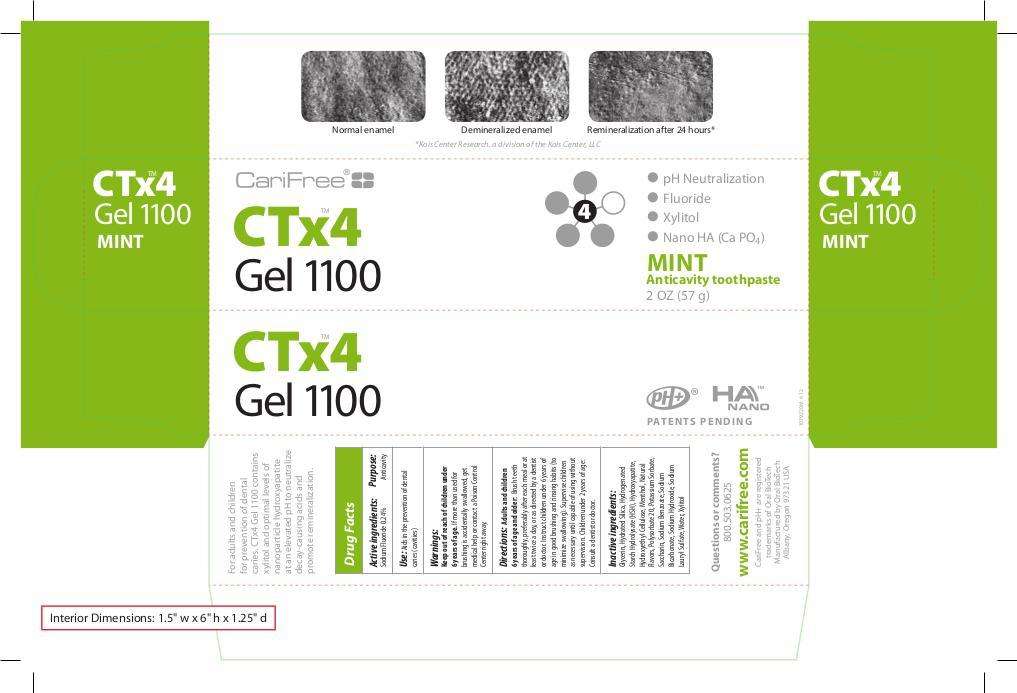
CTx4 Gel 1100
CariFree® CTx4 Gel 11000.24% w/w sodium fluoride
FULL PRESCRIBING INFORMATION
Sodium Fluoride 0.24%
Anticavity
Aids in the prevention of dental caries (cavities)
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Keep out of reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Adults and children 6 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor. Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing). Supervise children as necessary until capable of using without supervision. Children under 2 years of age: Consult a dentist or doctor.
Glycerin, Hydrated Silica, Hydrogenated Starch Hydrolysate (HSH), Hydroxyapatite, Hydroxyethyl Cellulose, Menthol (Mint only), Natural Flavors, Artificial Flavors (Citrus and Grape only) Polysorbate 20, Potassium Sorbate, Saccharin, Sodium Benzoate, Sodium Bicarbonate, Sodium Hydroxide, Sodium Lauryl Sulfate, Water, Xylitol

----------------------------------------------------------------------------------------------------
----------------------------------------------------------------------------------------------------

----------------------------------------------------------------------------------------------------

----------------------------------------------------------------------------------------------------

----------------------------------------------------------------------------------------------------

CTx4 Gel 1100sodium fluoride GEL, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CTx4 Gel 1100sodium fluoride GEL, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CTx4 Gel 1100sodium fluoride GEL, DENTIFRICE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||