Covergirl Advanced Radiance
Covergirl Advanced Radiance Age Defying Makeup + Sunscreen SPF 10 (All shades)
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Covergirl Advanced Radiance Uses
- Warnings
- Directions
- Covergirl Advanced Radiance Other information
- Inactive ingredients
- Questions?
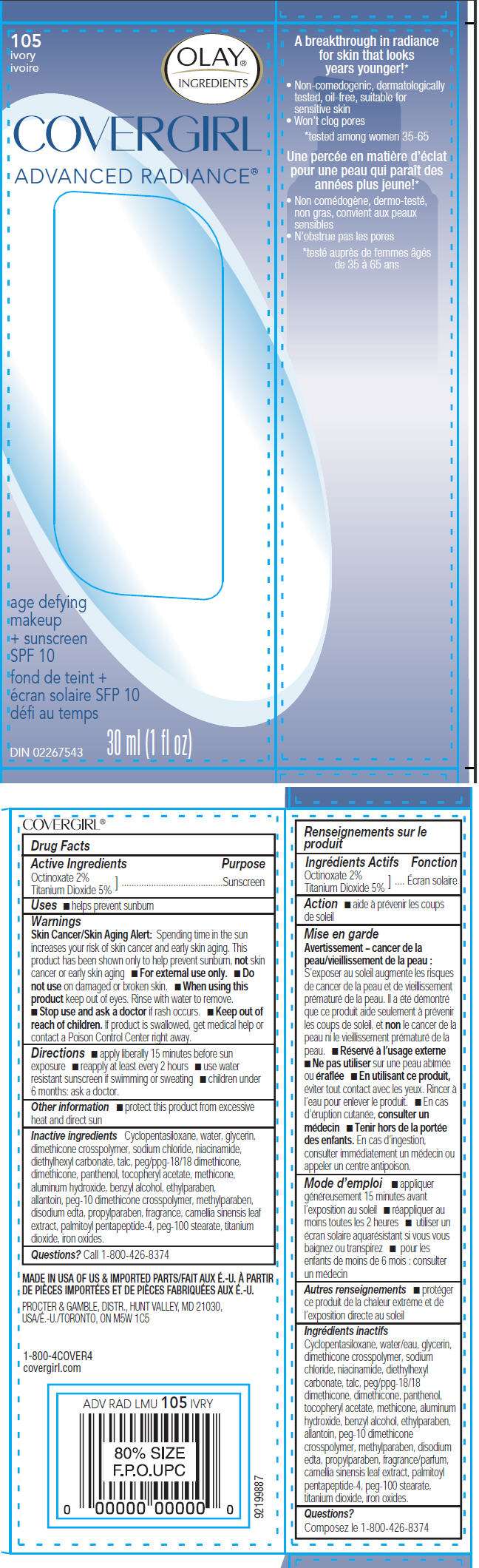
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredients
Octinoxate 2%
Titanium Dioxide 5%
Purpose
Sunscreen
Covergirl Advanced Radiance Uses
- helps prevent sunburn
Warnings
Skin Cancer/Skin Aging Alert
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging
- For external use only.
- Do not use on damaged or broken skin.
- When using this product keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if rash occurs.
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use water resistant sunscreen if swimming or sweating
- children under 6 months: ask a doctor.
Covergirl Advanced Radiance Other information
- protect this product from excessive heat and direct sun
Inactive ingredients
Cyclopentasiloxane, water, glycerin, dimethicone crosspolymer, sodium chloride, niacinamide, diethylhexyl carbonate, talc, peg/ppg-18/18 dimethicone, dimethicone, panthenol, tocopheryl acetate, methicone, aluminum hydroxide, benzyl alcohol, ethylparaben, allantoin, peg-10 dimethicone crosspolymer, methylparaben, disodium edta, propylparaben, fragrance, camellia sinensis leaf extract, palmitoyl pentapeptide-4, peg-100 stearate, titanium dioxide, iron oxides.
Questions?
Call 1-800-426-8374
PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
105
ivory
OLAY
®
INGREDIENTS
COVERGIRL
ADVANCED RADIANCE®
age defying
makeup
+ sunscreen
SPF 10
DIN 02267543
30 ml (1 fl oz)
Covergirl Advanced RadianceOctinoxate and Titanium Dioxide LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||