CONTINUOUS COVERAGE MAKEUP
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
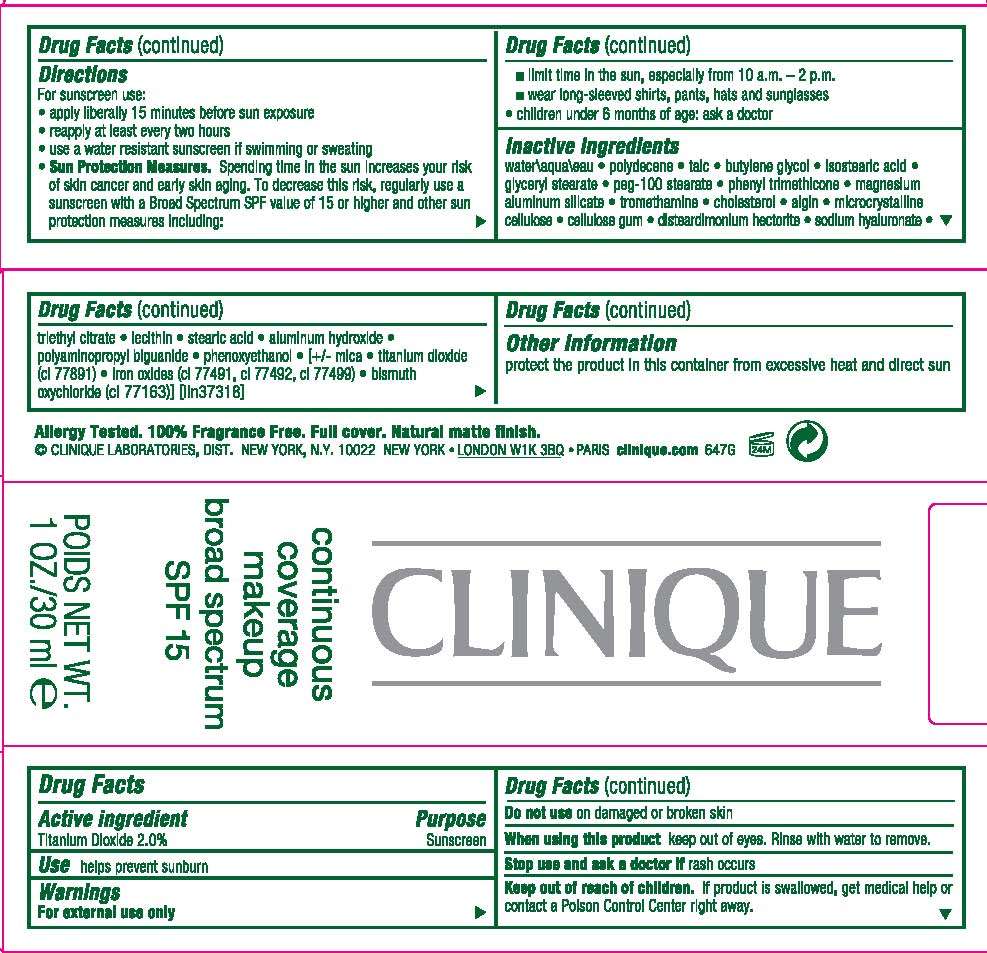
DRUG FACTS
ACTIVE INGREDIENT: 2% TITANIUM DIOXIDE
Purpose
WARNINGS:
FOR EXTERNAL USE ONLY
DO NOT USE ON DAMAGED OR BROKEN SKIN
WHEN USING THIS PRODUCT KEEP OUT OF EYES. RINSE WITH WATER TO REMOVE.
STOP USE AND ASK A DOCTOR IF RASH OCCURS
KEEP OUT OF REACH OF CHILDREN. IF PRODUCT IS SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
Uses
DIRECTIONS
- FOR SUNSCREEN USE:
- APPLY LIBERALLY 15 MINUTES BEFORE SUN EXPOSURE
- REAPPLY AT LEAST EVERY 2 HOURS
- USE A WATER RESISTANT SUNSCREEN IF SWIMMING OR SWEATING
- SUN PROTECTION MEASURES. SPENDING TIME IN THE SUN INCREASES YOUR RISK OF SKIN CANCER AND EARLY SKIN AGING. TO DECREASE THIS RISK, REGULARLY USE A SUNSCREEN WITH A BROAD SPECTRUM SPF VALUE OF 15 OR HIGHER AND OTHER SUN PROTECTION MEASURES INCLUDING:
-
- LIMIT TIME IN THE SUN, ESPECIALLY FROM 10 AM TO 2 PM
-
- WEAR LING-SLEEVED SHIRTS, PANTS AND SUNGLASSES
-
- CHILDREN UNDER 6 MONTHS OF AGE: ASK A DOCTOR
- CHILDREN UNDER 6 MONTHS OF AGE: ASK A DOCTOR
INACTIVE INGREDIENTS: water\aqua\eau [] polydecene [] talc [] butylene glycol [] isostearic acid [] glyceryl stearate [] peg-100 stearate [] phenyl trimethicone [] magnesium aluminum silicate [] tromethamine [] cholesterol [] algin [] microcrystalline cellulose [] cellulose gum [] disteardimonium hectorite [] sodium hyaluronate [] triethyl citrate [] lecithin [] stearic acid [] aluminum hydroxide [] polyaminopropyl biguanide [] phenoxyethanol [] [+/- mica [] titanium dioxide (ci 77891) [] iron oxides (ci 77491, ci 77492, ci 77499) [] bismuth oxychloride (ci 77163)
PROTECT THE PRODUCT IN THIS CONTAINER FROM EXCESSIVE HEAT AND DIRECT SUN
PRINCIPAL DISPLAY PANEL
CLINIQUE
CONTINUOUS
COVERAGE
MAKEUP
BROAD SPECTRUM
SPF 15
NET WT. 1 OZ/ 30 ML
CONTINUOUS COVERAGE MAKEUPTITANIUM DIOXIDE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||