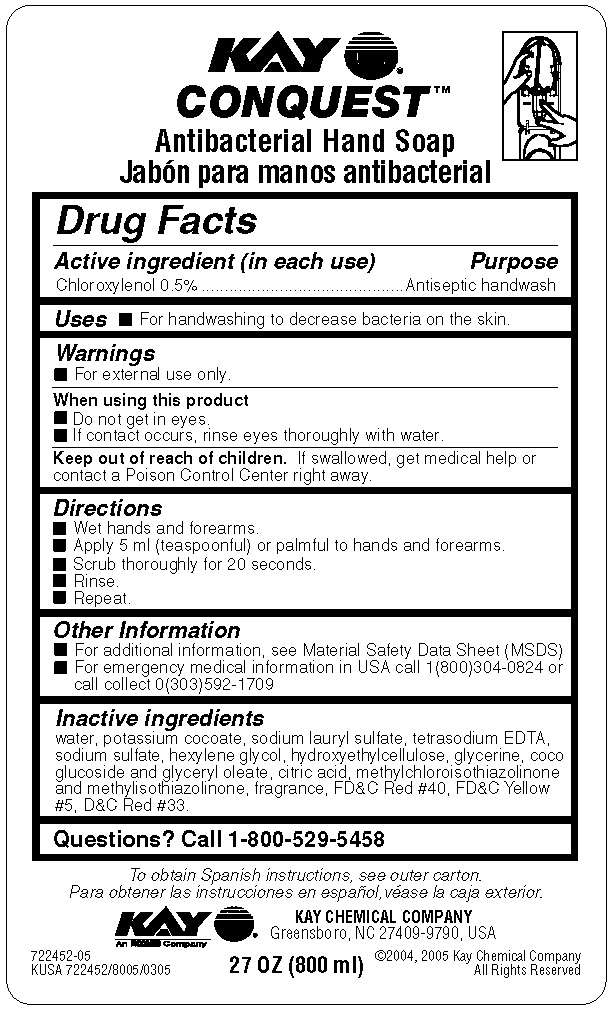
Conquest
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each use)
- Purpose
- Conquest Uses
- Warnings
- Directions
- Other Information
- Principal display panel and representative label
FULL PRESCRIBING INFORMATION
Active ingredient (in each use)
Chloroxylenol 0.5%
Purpose
Antiseptic handwash
Conquest Uses
- For handwashing to decrease bacteria on the skin.
Warnings
- For external use only.
When using this product
- Do not get in eyes.
- If contact occurs, rinse eyes thoroughly with water.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Wet hands and forearms.
- Apply 5 ml (teaspoonful) or palmful to hands and forearms.
- Scrub thoroughly for 20 seconds.
- Rinse.
- Repeat.
Other Information
- For additional information, see Material Safety Data Sheet (MSDS)
- For emergency medical information in USA call 1(800)304-0824 or call collect 0(303)592-1709
Inactive ingredients water, potassium cocoate, sodium lauryl sulfate, tetrasodium EDTA, sodium sulfate, hexylene glycol, hydroxyethylcellulose, glycerine, coco glucoside and glyceryl oleate, citric acid, methylchloroisothiazolinone and methylisothiazolinone, fragrance, FDC Red #40, FDC Yellow #5, DC Red #33
Questions? Call 1-800-529-5458
Principal display panel and representative label
KAY
CONQUEST
Antibacterial hand Soap
KAY
An ECOALB Company
KAY CHEMICAL COMPANY
Greensboro, NC 27409-9790, USA
27 OZ (800 ml)
722452-05
KUSA 722452/8005/0305
copyright 2004, 2005 Kay Chemical Company All Rights Reserved
ConquestChloroxylenol SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||