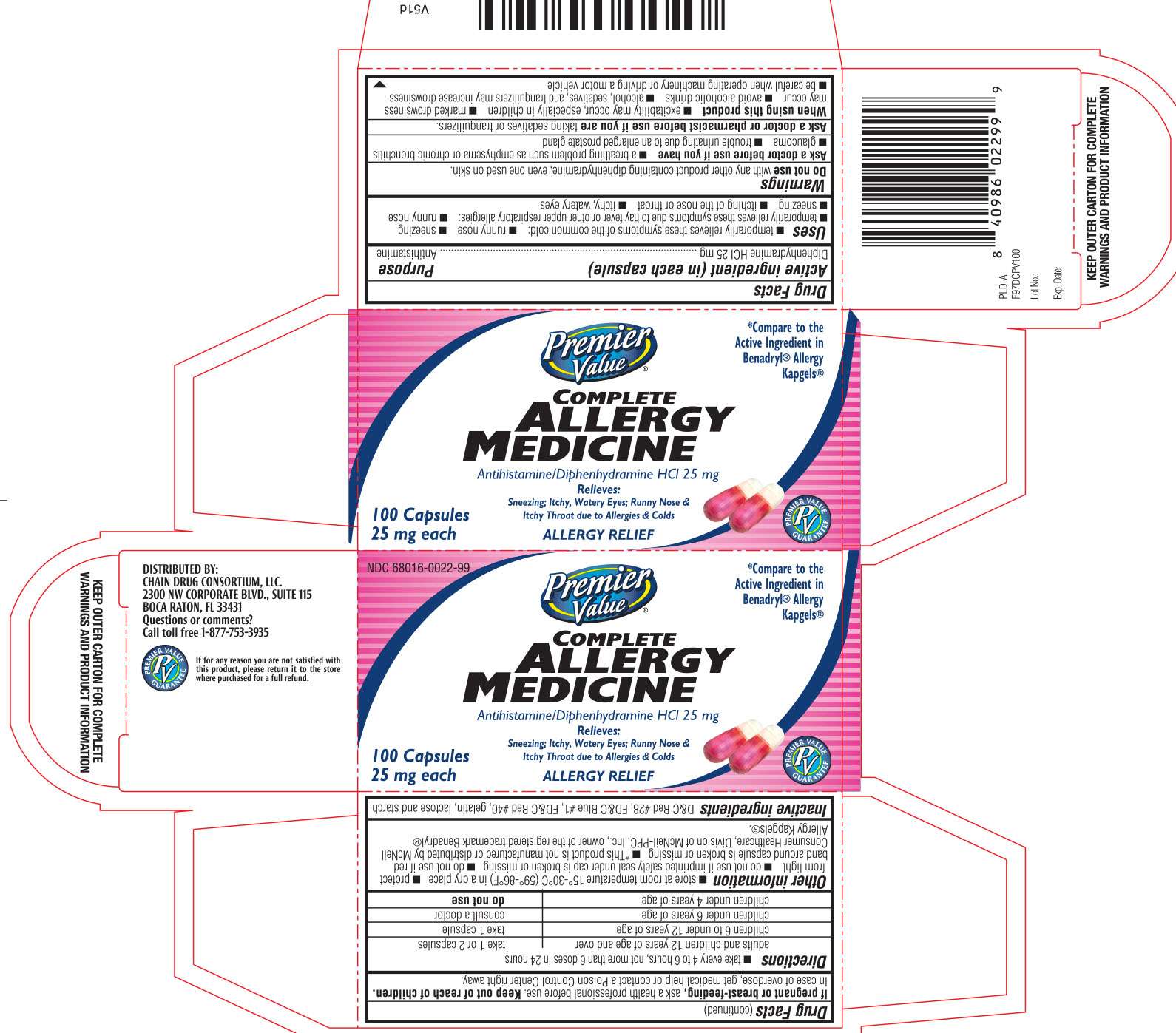
Complete Allergy Medicine
Chain Drug Consortium, LLC (Premier Value)
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each capsule)
- Purpose
- Complete Allergy Medicine Uses
- Warnings
- Directions
- Complete Allergy Medicine Other information
- Inactive ingredients
- Principal Display Panel
- Product Label
FULL PRESCRIBING INFORMATION
Active ingredient (in each capsule)
Diphenhydramine HCl 25 mg
Purpose
Antihistamine
Complete Allergy Medicine Uses
-
temporarily relieves these symptoms of the common cold:
-
runny nose
-
sneezing
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
-
runny nose
-
sneezing
-
itching of the nose or throat
-
itchy, watery eyes
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
-
a breathing problem such as emphysema or chronic bronchitis
-
glaucoma
-
trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
-
excitability may occur, especially in children
-
marked drowsiness may occur
-
avoid alcoholic drinks
-
alcohol, sedatives, and tranquilizers may increase drowsiness
-
be careful when driving a motor vehicle or operating machinery
If pregnant of breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
-
take every 4 to 6 hours, not take more than 6 doses in 24 hours
| adults and children 12 years of age and over | take 1 or 2 capsules |
| children 6 to under 12 years of age | take 1 capsule |
| children under 6 years of age | consult a doctor |
| children under 4 years of age | do not use |
Complete Allergy Medicine Other information
-
store at controlled room temperature 15°-30°C (59°-86°F) in a dry place
-
protect from light
-
do not use if imprinted safety seal under cap is broken or missing
-
*This product is not manufactured or distributed by McNeil Consumer Healthcare, Division of McNeil- PPC, Inc., owner of the registered trademark Benadryl® Allergy Kapgels®
Inactive ingredients
D&C red #28, FD&C blue #1, FD&C red #40, gelatin, lactose, starch
Principal Display Panel
Premier Value
Complete Allergy Medicine
Antihistamine/ Diphenhydramine HCl 25 mg
Relieves:
sneezing; itchy, watery eyes; runny nose & itchy throat due to allergies & colds
*Compare to the Active Ingredient in Benadryl® Allergy Kapgels®
Distributed by:
Chain Drug Consortium, LLC.
2300 NW Corporate BLVD., Suite 115
Boca Raton, FL 33431
Questions or comments?
Call toll free 1-877-753-3935
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
Product Label
Complete Allergy MedicineDiphenhydramine HCl CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||