COLESTIPOL HYDROCHLORIDE
Global Pharmaceuticals, Division of Impax Laboratories Inc.
Colestipol Hydrochloride for Oral Suspension, USP 5 grams/packet and 5 grams/scoopful
FULL PRESCRIBING INFORMATION: CONTENTS*
- COLESTIPOL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- COLESTIPOL HYDROCHLORIDE INDICATIONS AND USAGE
- COLESTIPOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- COLESTIPOL HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- COLESTIPOL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL - 30 Packet Carton
- PRINCIPAL DISPLAY PANEL - 500 gram Bottle
FULL PRESCRIBING INFORMATION
Rx only
COLESTIPOL HYDROCHLORIDE DESCRIPTION
Colestipol hydrochloride for oral suspension USP contains colestipol hydrochloride, which is a lipid lowering agent for oral use. Colestipol hydrochloride is an insoluble, high molecular weight basic anion-exchange copolymer of diethylenetriamine and 1-chloro-2, 3-epoxypropane, with approximately 1 out of 5 amine nitrogens protonated (chloride form). It is a light yellow water-insoluble resin which is hygroscopic and swells when suspended in water or aqueous fluids.
Colestipol HCl for oral suspension USP is tasteless and odorless. The inactive ingredient is silicon dioxide. One dose (1 packet or 1 level scoopful) of colestipol HCl for oral suspension USP contains 5 grams of colestipol hydrochloride.
CLINICAL PHARMACOLOGY
Cholesterol is the major, and probably the sole precursor of bile acids. During normal digestion, bile acids are secreted via the bile from the liver and gall bladder into the intestines. Bile acids emulsify the fat and lipid materials present in food, thus facilitating absorption. A major portion of the bile acids secreted is reabsorbed from the intestines and returned via the portal circulation to the liver, thus completing the enterohepatic cycle. Only very small amounts of bile acids are found in normal serum.
Colestipol hydrochloride binds bile acids in the intestine forming a complex that is excreted in the feces. This nonsystemic action results in a partial removal of the bile acids from the enterohepatic circulation, preventing their reabsorption. Since colestipol hydrochloride is an anion exchange resin, the chloride anions of the resin can be replaced by other anions, usually those with a greater affinity for the resin than chloride ion.
Colestipol hydrochloride is hydrophilic, but it is virtually water insoluble (99.75%) and it is not hydrolyzed by digestive enzymes. The high molecular weight polymer in colestipol hydrochloride apparently is not absorbed. In humans, less than 0.17% of a single 14C-labeled colestipol hydrochloride dose is excreted in the urine when given following 60 days of chronic dosing of 20 grams of colestipol hydrochloride per day.
The increased fecal loss of bile acids due to colestipol hydrochloride administration leads to an increased oxidation of cholesterol to bile acids. This results in an increase in the number of low-density lipoprotein (LDL) receptors, increased hepatic uptake of LDL and a decrease in beta lipoprotein or low density lipoprotein serum levels, and a decrease in serum cholesterol levels. Although colestipol hydrochloride produces an increase in the hepatic synthesis of cholesterol in man, serum cholesterol levels fall.
There is evidence to show that this fall in cholesterol is secondary to an increased rate of clearance of cholesterol-rich lipoproteins (beta or low density lipoproteins) from the plasma. Serum triglyceride levels may increase or remain unchanged in colestipol hydrochloride treated patients.
The decline in serum cholesterol levels with colestipol hydrochloride treatment is usually evident by one month. When colestipol hydrochloride is discontinued, serum cholesterol levels usually return to baseline levels within one month. Periodic determinations of serum cholesterol levels as outlined in the National Cholesterol Education Program (NCEP) guidelines should be done to confirm a favorable initial and long-term response1.
In a large, placebo-controlled, multiclinic study, the LRC-CPPT,2 hypercholesterolemic subjects treated with cholestyramine, a bile-acid sequestrant with a mechanism of action and an effect on serum cholesterol similar to that of colestipol hydrochloride, had reductions in total and low-density lipoprotein cholesterol (LDL-C). Over the seven-year study period the cholestyramine group experienced a 19% reduction (relative to the incidence in the placebo group) in the combined rate of coronary heart disease death plus non-fatal myocardial infarction (cumulative incidences of 7% cholestyramine and 8.6%, placebo). The subjects included in the study were middle-aged men (age 35-59) with serum cholesterol-levels above 265 mg/dL, LDL-C above 175 mg/dL on a moderate cholesterol-lowering diet, and no history of heart disease. It is not clear to what extent these findings can be extrapolated to other segments of the hypercholesterolemic population not studied.
Treatment with colestipol hydrochloride results in a significant increase in lipoprotein LpAI. Lipoprotein LpAI is one of the two major lipoprotein particles within the high-density lipoprotein (HDL) density range3, and has been shown in cell culture to promote cholesterol efflux or removal from cells4. Although the significance of this finding has not been established in clinical studies, the elevation of the lipoprotein LpAI particle within the HDL fraction is consistent with an antiatherogenic effect of colestipol hydrochloride, even though little change is observed in HDL cholesterol.
In patients with heterozygous familial hypercholesterolemia who have not obtained an optimal response to colestipol hydrochloride alone in maximal doses, the combination of colestipol hydrochloride and nicotinic acid has been shown to further lower serum cholesterol, triglyceride, and LDL cholesterol (LDL-C) values. Simultaneously, HDL cholesterol (HDL-C) values increased significantly. In many such patients it is possible to normalize serum lipid values.5-7
Preliminary evidence suggests that the cholesterol-lowering effects of lovastatin and the bile acid sequestrant, colestipol hydrochloride, are additive.
The effect of intensive lipid-lowering therapy on coronary atherosclerosis has been assessed by arteriography in hyperlipidemic patients. In these randomized, controlled clinical trials, patients were treated for two to four years by either conventional measures (diet, placebo, or in some cases low-dose resin), or with intensive combination therapy using diet and colestipol HCl for oral suspension plus either nicotinic acid or lovastatin. When compared to conventional measures, intensive lipid-lowering combination therapy significantly reduced the frequency of progression and increased the frequency of regression of coronary atherosclerotic lesions in patients with or at risk for coronary artery disease.8-11
COLESTIPOL HYDROCHLORIDE INDICATIONS AND USAGE
Since no drug is innocuous, strict attention should be paid to the indications and contraindications, particularly when selecting drugs for chronic long-term use.
Colestipol HCl for oral suspension USP is indicated as adjunctive therapy to diet for the reduction of elevated serum total and low-density lipoprotein (LDL) cholesterol in patients with primary hypercholesterolemia (elevated low density lipoproteins [LDL] cholesterol) who do not respond adequately to diet. Generally, colestipol HCl for oral suspension USP has no clinically significant effect on serum triglycerides, but with its use triglyceride levels may be raised in some patients.
Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Treatment should begin and continue with dietary therapy (see NCEP guidelines). A minimum of six months of intensive dietary therapy and counseling should be carried out prior to initiation of drug therapy. Shorter periods may be considered in patients with severe elevations of LDL-C or with definite CHD.
According to the NCEP guidelines, the goal of treatment is to lower LDL-C, and LDL-C is to be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the Total-C be used to monitor therapy. The NCEP treatment guidelines are shown below.
| LDL-Cholesterol mg/dL (mmol/L) |
|||
|---|---|---|---|
| Definite Atheroschlerotic Disease |
Two or More Other Risk Factors |
Initiation Level | Goal |
| No | No | ≥190 (≥4.9) |
<160 (<4.1) |
| No | Yes | ≥160 (≥4.1) |
<130 (<3.4) |
| Yes | Yes or No | ≥130 (≥3.4) |
≤100 (≤2.6) |
COLESTIPOL HYDROCHLORIDE CONTRAINDICATIONS
Colestipol HCl for oral suspension is contraindicated in those individuals who have shown hypersensitivity to any of its components.
WARNINGS
TO AVOID ACCIDENTAL INHALATION OR ESOPHAGEAL DISTRESS, COLESTIPOL HCL FOR ORAL SUSPENSION SHOULD NOT BE TAKEN IN ITS DRY FORM. ALWAYS MIX COLESTIPOL HCL FOR ORAL SUSPENSION WITH WATER OR OTHER FLUIDS BEFORE INGESTING.
PRECAUTIONS
Prior to initiating therapy with colestipol HCl for oral suspension, secondary causes of hypercholesterolemia (e.g., poorly controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dysproteinemias, obstructive liver disease, other drug therapy, alcoholism), should be excluded, and a lipid profile performed to assess Total cholesterol, HDL-C, and triglycerides (TG). For individuals with TG less than 400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation:
LDL-C = Total cholesterol - [ (Triglycerides / 5)+HDL-C]
For TG levels >400 mg/dL, this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation. In hypertriglyceridemic patients, LDL-C may be low or normal despite elevated Total-C. In such cases colestipol HCl for oral suspension may not be indicated.
Because it sequesters bile acids, colestipol hydrochloride may interfere with normal fat absorption and thus may reduce absorption of folic acid and fat soluble vitamins such as A, D, and K.
Chronic use of colestipol hydrochloride may be associated with an increased bleeding tendency due to hypoprothrombinemia from vitamin K deficiency. This will usually respond promptly to parenteral vitamin K1 and recurrences can be prevented by oral administration of vitamin K1.
Serum cholesterol and triglyceride levels should be determined periodically based on NCEP guidelines to confirm a favorable initial and adequate long-term response.
Colestipol HCl for oral suspension may produce or severely worsen pre-existing constipation. The dosage should be increased gradually in patients to minimize the risk of developing fecal impaction. In patients with pre-existing constipation, the starting dose should be 1 packet or 1 scoop once daily for 5-7 days, increasing to twice daily with monitoring of constipation and of serum lipoproteins, at least twice, 4-6 weeks apart. Increased fluid and fiber intake should be encouraged to alleviate constipation and a stool softener may occasionally be indicated. If the initial dose is well tolerated, the dose may be increased as needed by one dose/day (at monthly intervals) with periodic monitoring of serum lipoproteins. If constipation worsens or the desired therapeutic response is not achieved at one to six doses/day, combination therapy or alternate therapy should be considered. Particular effort should be made to avoid constipation in patients with symptomatic coronary artery disease. Constipation associated with colestipol HCl for oral suspension may aggravate hemorrhoids.
While there have been no reports of hypothyroidism induced in individuals with normal thyroid function, the theoretical possibility exists, particularly in patients with limited thyroid reserve.
Since colestipol hydrochloride is a chloride form of an anion exchange resin, there is a possibility that prolonged use may lead to the development of hyperchloremic acidosis.
Carcinogenesis, mutagenesis and impairment of fertility
In studies conducted in rats in which cholestyramine resin (a bile acid sequestering agent similar to colestipol hydrochloride) was used as a tool to investigate the role of various intestinal factors, such as fat, bile salts and microbial flora, in the development of intestinal tumors induced by potent carcinogens, the incidence of such tumors was observed to be greater in cholestyramine resin treated rats than in control rats.
The relevance of this laboratory observation from studies in rats with cholestyramine resin to the clinical use of colestipol hydrochloride is not known. In the LRC-CPPT study referred to above, the total incidence of fatal and non-fatal neoplasms was similar in both treatment groups. When the many different categories of tumors are examined, various alimentary system cancers were somewhat more prevalent in the cholestyramine group. The small numbers and the multiple categories prevent conclusions from being drawn. Further follow-up of the LRC-CPPT participants by the sponsors of that study is planned for cause-specific mortality and cancer morbidity.
When colestipol hydrochloride was administered in the diet to rats for 18 months, there was no evidence of any drug related intestinal tumor formation. In the Ames assay, colestipol hydrochloride was not mutagenic.
Use in Pregnancy
Since colestipol hydrochloride is essentially not absorbed systemically (less than 0.17% of the dose), it is not expected to cause fetal harm when administered during pregnancy in recommended dosages. There are no adequate and well controlled studies in pregnant women, and the known interference with absorption of fat soluble vitamins may be detrimental even in the presence of supplementation. The use of colestipol HCl for oral suspension in pregnancy or by women of childbearing potential requires that the potential benefits of drug therapy be weighed against possible hazards to the mother or child.
Nursing Mother
Caution should be exercised when colestipol HCl for oral suspension is administered to a nursing mother. The possible lack of proper vitamin absorption described in the "pregnancy" section may have an effect on nursing infants.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established.
Drug Interactions
Since colestipol hydrochloride is an anion exchange resin, it may have a strong affinity for anions other than the bile acids. In vitro studies have indicated that colestipol hydrochloride binds a number of drugs. Therefore, colestipol HCl for oral suspension resin may delay or reduce the absorption of concomitant oral medication. The interval between the administration of colestipol HCl for oral suspension and any other medication should be as long as possible. Patients should take other drugs at least one hour before or four hours after colestipol HCl for oral suspension to avoid impeding their absorption.
Repeated doses of colestipol hydrochloride given prior to a single dose of propranolol in human trials have been reported to decrease propranolol absorption. However, in a follow-up study in normal subjects, single dose administration of colestipol hydrochloride and propranolol and twice-a-day administration for 5 days of both agents did not effect the extent of propranolol absorption, but had a small yet statistically significant effect on its rate of absorption; the time to reach maximum concentration was delayed 30 minutes. Effects on the absorption of other beta-blockers have not been determined. Therefore, patients on propranolol should be observed when colestipol HCl for oral suspension is either added or deleted from a therapeutic regimen.
Studies in humans show that the absorption of chlorothiazide as reflected in urinary excretion is markedly decreased even when administered one hour before colestipol hydrochloride. The absorption of tetracycline, furosemide, penicillin G, hydrochlorothiazide, and gemfibrozil was significantly decreased when given simultaneously with colestipol hydrochloride; these drugs were not tested to determine the effect of administration one hour before colestipol hydrochloride.
No depressant effect on blood levels in humans was noted when colestipol hydrochloride was administered with any of the following drugs: aspirin, clindamycin, clofibrate, methyldopa, nicotinic acid (niacin), tolbutamide, phenytoin or warfarin. Particular caution should be observed with digitalis preparations since there are conflicting results for the effect of colestipol hydrochloride on the availability of digoxin and digitoxin. The potential for binding of these drugs if given concomitantly is present. Discontinuing colestipol hydrochloride could pose a hazard to health if a potentially toxic drug that is significantly bound to the resin has been titrated to a maintenance level while the patient was taking colestipol hydrochloride.
Bile acid binding resins may also interfere with the absorption of oral phosphate supplements and hydrocortisone.
COLESTIPOL HYDROCHLORIDE ADVERSE REACTIONS
Gastrointestinal
The most common adverse reactions are confined to the gastrointestinal tract. To achieve minimal GI disturbance with an optimal LDL-cholesterol lowering effect, a gradual increase of dosage starting with one dose/day is recommended. Constipation is the major single complaint and at times is severe. Most instances of constipation are mild, transient, and controlled with standard treatment. Increased fluid intake and inclusion of additional dietary fiber should be the first step; a stool softener may be added if needed. Some patients require decreased dosage or discontinuation of therapy. Hemorrhoids may be aggravated.
Other, less frequent gastrointestinal complaints consist of abdominal discomfort (abdominal pain and cramping), intestinal gas, (bloating and flatulence), indigestion and heartburn, diarrhea and loose stools, and nausea and vomiting. Bleeding hemorrhoids and blood in the stool have been infrequently reported. Peptic ulceration, cholecystitis, and cholelithiasis have been rarely reported in patients receiving colestipol HCl for oral suspension, and are not necessarily drug related.
Transient and modest elevations of aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT) and alkaline phosphatase were observed on one or more occasions in various patients treated with colestipol hydrochloride.
The following non-gastrointestinal adverse reactions have been reported with generally equal frequency in patients receiving colestipol HCl for oral suspension or placebo in clinical studies:
Cardiovascular
Chest pain, angina, and tachycardia have been infrequently reported.
Hypersensitivity
Rash has been infrequently reported. Urticaria and dermatitis have been rarely noted in patients receiving colestipol HCl for oral suspension.
Musculoskeletal
Musculoskeletal pain, aches and pains in the extremities, joint pains, arthritis, and backache have been reported.
Neurologic
Headache, migraine headache and sinus headache have been reported. Other infrequently reported complaints include dizziness, light-headedness, and insomnia.
Miscellaneous
Anorexia, fatigue, weakness, shortness of breath, and swelling of the hands or feet, have been infrequently reported.
OVERDOSAGE
Overdosage of colestipol HCl for oral suspension has not been reported. Should overdosage occur, however, the chief potential harm would be obstruction of the gastrointestinal tract. The location of such potential obstruction, the degree of obstruction and the presence or absence of normal gut motility would determine treatment.
COLESTIPOL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
One dose (1 packet or 1 level scoopful) of colestipol HCl for oral suspension contains 5 gram of colestipol hydrochloride. The recommended daily adult dose is one to six packets or level scoopfuls given once or in divided doses. Treatment should be started with one dose once or twice daily with an increment of one dose/day at one- or two-month intervals. Appropriate use of lipid profiles as per NCEP guidelines including LDL-cholesterol and triglycerides is advised so that optimal, but not excessive doses are used to obtain the desired therapeutic effect on LDL-cholesterol level. If the desired therapeutic effect is not obtained at one to six doses/day with good compliance and acceptable side effects, combined therapy or alternate treatment should be considered.
To avoid accidental inhalation or esophageal distress, colestipol HCl for oral suspension should not be taken in its dry form. Colestipol HCl for oral suspension should always be mixed with water or other fluids before ingesting. Patients should take other drugs at least one hour before or four hours after colestipol HCl for oral suspension to minimize possible interference with their absorption. (See PRECAUTIONS, Drug Interactions.)
The scoop accompanying this product is not interchangeable with other scoops.
Before Colestipol HCl for Oral Suspension Administration
- Define the type of hyperlipoproteinemia, as described in NCEP guidelines.
- Institute a trial of diet and weight reduction.
- Establish baseline serum total and LDL-cholesterol and triglyceride levels.
During Colestipol HCl for Oral Suspension Administration
- The patient should be carefully monitored clinically, including serum cholesterol and triglyceride levels. Periodic determinations of serum cholesterol levels as outlined in the NCEP guidelines should be done to confirm a favorable initial and longer term response.
- Failure of total or LDL-cholesterol to fall within the desired range should lead one to first examine dietary and drug compliance. If these are deemed acceptable, combined therapy or alternate treatment should be considered.
- Significant rise in triglyceride level should be considered as indication for dose reduction, drug discontinuation, or combined or alternate therapy.
Mixing and Administration Guide
Colestipol HCl for oral suspension should always be mixed in a liquid such as water or the beverage of your choice. It may also be taken in soups or with cereals or pulpy fruits. Colestipol HCl for oral suspension should never be taken in its dry form.
With Beverages
- Add the prescribed amount of colestipol HCl for oral suspension to a glassful (three ounces or more) of water or the beverage of your choice. A heavy or pulpy juice may minimize complaints relative to consistency.
- Stir the mixture until the medication is completely mixed. (Colestipol HCl for oral suspension will not dissolve in the liquid.) Colestipol HCl for oral suspension may also be mixed with carbonated beverages, slowly stirred in a large glass; however, this mixture may be associated with GI complaints.
Rinse the glass with a small amount of additional beverage to make sure all the medication is taken.
With cereals, soups, and fruits
Colestipol HCl for oral suspension may be taken mixed with milk in hot or regular breakfast cereals, or even mixed in soups that have a high fluid content. It may also be added to fruits that are pulpy such as crushed pineapple, pears, peaches, or fruit cocktail.
HOW SUPPLIED
Colestipol HCl for oral suspension USP is available as follows:
| Carton of 30 foil packets | NDC 0115-5212-18 |
| Carton of 90 foil packets | NDC 0115-5212-29 |
| Bottle of 500 grams with scoop | NDC 0115-5213-02 |
Each packet or level scoop supplies 5 grams of colestipol HCl for oral suspension USP.
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
REFERENCES
- Summary of the Second Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). JAMA 269(23):3015-3023, 1993.
- Lipid Metabolism-Atherogenesis Branch, National Heart, Lung, and Blood Institute, Bethesda, MD: The Lipid Research Clinics Coronary Primary Prevention Trial Results. I . Reduction in Incidence of Coronary Heart Disease. JAMA 251:351-364, 1984.
- Parra HJ, et al. Differential electroimmunoassay of human LpA-I lipoprotein particles on ready-to-use plates. Clin. Chem. 36(8):1431-1435, 1990.
- Barbaras R, et al. Cholesterol efflux from cultured adipose cells is mediated by LpAI particles but not by LpAI:AII particles. Biochem. Biophys. Res. Comm. 142(1):63-69, 1987.
- Kane JP, et al. Normalization of low-density-lipoprotein levels in heterozygous familial hypercholesterolemia with a combined drug regimen. N Engl. J. Med. 304:251-258, 1981.
- Illingworth DR, et al. Colestipol plus nicotinic acid in treatment of heterozygous familial hypercholesterolemia. Lancet 1:296-298, 1981.
- Kuo PT, et al. Familial type II hyperlipoproteinemia with coronary heart disease: Effect of diet-colestipol-nicotinic acid treatment. Chest 79:286-291, 1981.
- Blankenhorn DH, et al. Beneficial Effects of Combined Colestipol-Niacin Therapy on Coronary Atherosclerosis and Coronary Venous Bypass Grafts. JAMA 257(23):3233-3240, 1987.
- Cashin-Hemphill L, et al. Beneficial Effects of Colestipol-Niacin on Coronary Atherosclerosis: A 4-Year Follow-up. JAMA 264:3013-3017, 1990.
- Brown G, et al. Regression of Coronary Artery Disease as a Result of Intensive Lipid-Lowering Therapy in Men with High Levels of Apolipoprotein B. N Engl, J. Med 323:1289-1298, 1990.
- Kane JP, et al. Regression of Coronary Atherosclerosis During Treatment of Familial Hypercholesterolemia with Combined Drug Regimens. JAMA 264:3007-3012, 1990.
Dist. by:
Global Pharmaceuticals
Division of IMPAX Laboratories, Inc.
Philadelphia, PA 19124 USA
537-02
Rev. 03/2013
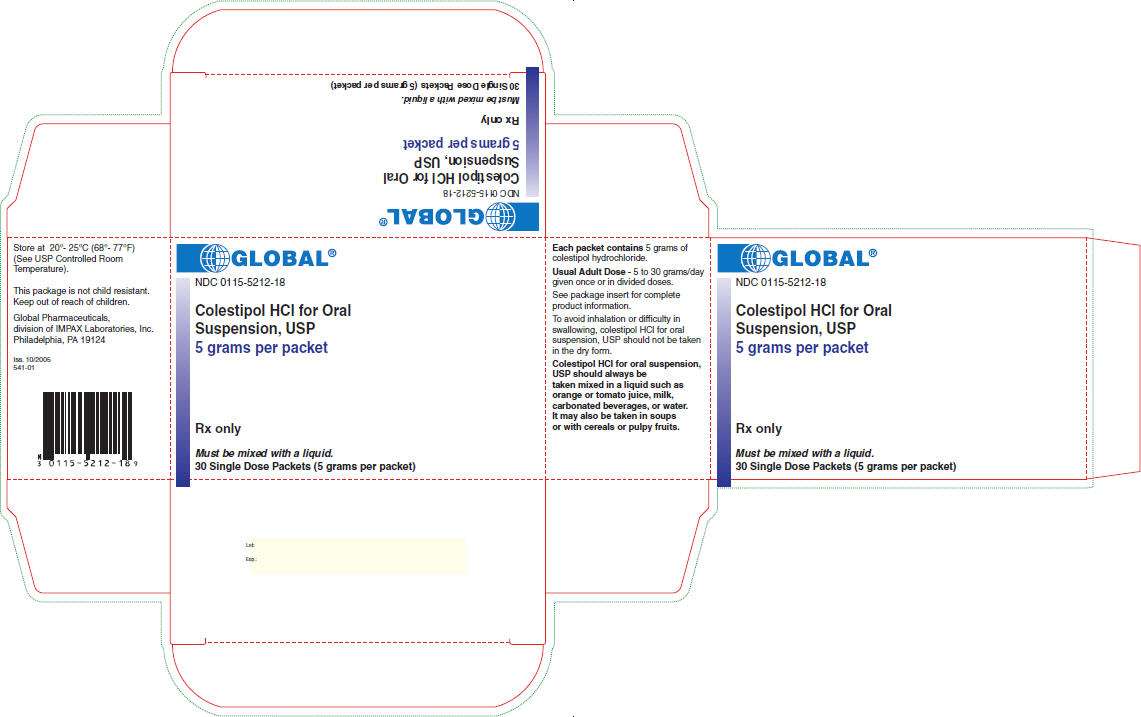
PRINCIPAL DISPLAY PANEL - 30 Packet Carton
GLOBAL®
NDC 0115-5212-18
Colestipol HCl for Oral
Suspension, USP
5 grams per packet
Rx only
Must be mixed with a liquid.
30 Single Dose Packets (5 grams per packet)
PRINCIPAL DISPLAY PANEL - 500 gram Bottle
GLOBAL®
NDC 0115-5213-02
Colestipol HCl for Oral
Suspension, USP
5 grams per scoopful
Rx only
Must be mixed with a liquid.
Net quantity 500 grams granules (17.6 oz)

COLESTIPOL HYDROCHLORIDECOLESTIPOL HYDROCHLORIDE SUSPENSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COLESTIPOL HYDROCHLORIDECOLESTIPOL HYDROCHLORIDE SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||