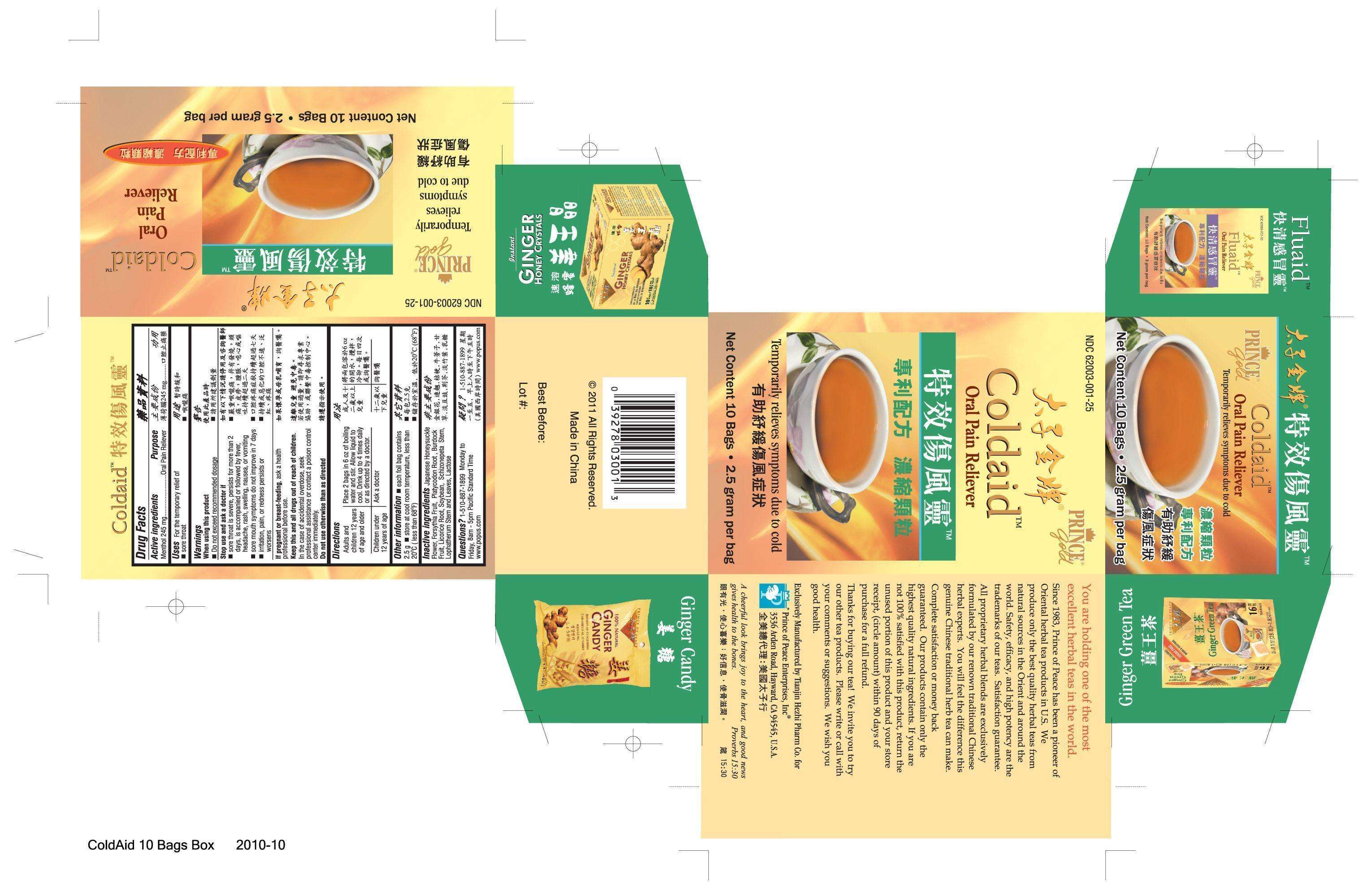
Coldaid
PRINCE OF PEACE ENTERPRISES INC.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients Purpose
Menthol 245 mg...........................Oral Pain Reliever
Purpose
Uses For the temporary relief of sore throat
When using this product
- Do not exceed recommended dosage
Stop use and ask a doctor if
- sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
If pregnant or breast-feeding, ask a health professional before use.
Keep this and all drugs out of reach of children.
- Questions? 1-510-887-1899 Monday to Friday, 8 am - 5 pm Pacific Standard Time
- www.popus.com
Directions
| Adults and children 12 years of age and older |
Place 2 bags in 6 oz of boiling water and stir. Allow liquid to cool. Drink up to 4 times daily or as directed by a doctor. |
| Children under 12 years of age |
Ask a doctor |
In the case of accidental overdose, seek professional assistance or contact a poison control center immediately.
Do not use otherwise than as directed.
Uses
Uses For the temporary relief of sore throat.
Japanese Hondysuckle Flower, Forsythia Fruit, Platycodon Root, Burdock Fruit, Licorice Root, Soybean, Schizonepeta Stem, Lophatherum Stem and Leaves, Lactose
Coldaidmenthol GRANULE, FOR SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||