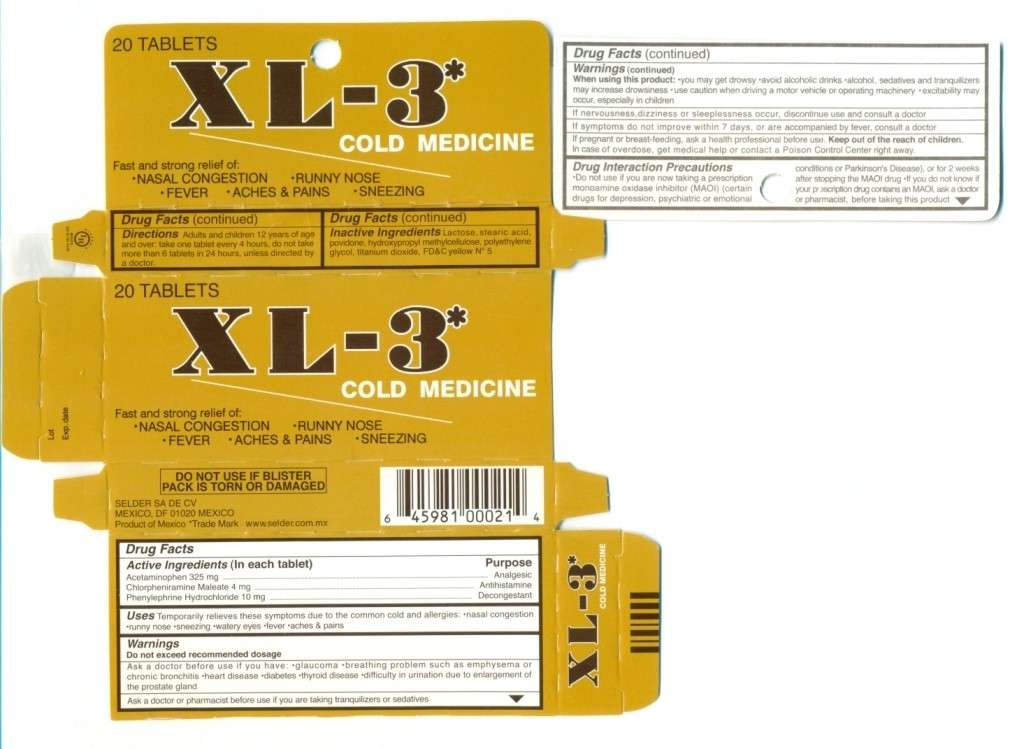
Cold Medicine
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients (in each capsule)Purpose
PurposeUses
Uses WarningsDo exceedrecommended dosage
Keep this and all drugs out of the reach of children. I
Directions Adults and children 12 years of age and older; take 1 tablet every 4 hours, do not take more than 6 tablets in 24 hours, unless directed by a doctor.
Inactive Ingredients Lactose, stearic acid, povidone, hydroxypropylmethylcellulose, polyethylene glycol, titanium dioxide, FDC Yellow No. 5
Cold MedicineAcetaminophen Chlorpheniramine Maleate Phenylepherine Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!