Clonidine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLONIDINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- CLONIDINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- CLONIDINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
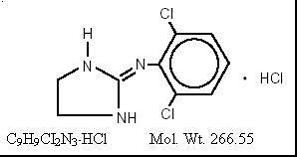
CLONIDINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Pharmacokinetics:The plasma level of clonidine peaks in approximately 3 to 5 hours and the plasma half-life ranges from 12 to 16 hours. The half-life increases up to 41 hours in patients with severe impairment of renal function. Following oral administration about 40 to 60% of the absorbed dose is recovered in the urine as unchanged drug in 24 hours. About 50% of the absorbed dose is metabolized in the liver. Neither food nor the race of the patient influences the pharmacokinetics of clonidine.
INDICATIONS & USAGE
CLONIDINE HYDROCHLORIDE CONTRAINDICATIONS
PRECAUTIONS).WARNINGS
Withdrawal:Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and tremor accompanied or followed by a rapid rise in blood pressure and elevated catecholamine concentrations in the plasma. The likelihood of such reactions to discontinuation of clonidine therapy appears to be greater after administration of higher doses or continuation of concomitant beta-blocker treatment and special caution is therefore advised in these situations. Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after clonidine withdrawal. When discontinuing therapy with clonidine hydrochloride tablets, USP, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal symptomatology.An excessive rise in blood pressure following discontinuation of clonidine therapy can be reversed by administration of oral clonidine hydrochloride or by intravenous phentolamine. If therapy is to be discontinued in patients receiving a beta-blocker and clonidine concurrently, the beta-blocker should be withdrawn several days before the gradual discontinuation of clonidine hydrochloride tablets.
Because children commonly have gastrointestinal illnesses that lead to vomiting, they may be particularly susceptible to hypertensive episodes resulting from abrupt inability to take medication.
PRECAUTIONS
GeneralIn patients who have developed localized contact sensitization to transdermal clonidine, continuation of transdermal clonidine or substitution of oral clonidine hydrochloride therapy may be associated with the development of a generalized skin rash.
In patients who develop an allergic reaction to transdermal clonidine, substitution of oral clonidine hydrochloride may also elicit an allergic reaction (including generalized rash, urticaria, or angioedema).
Clonidine hydrochloride tablets should be used with caution in patients with severe coronary insufficiency, conduction disturbances, recent myocardial infarction, cerebrovascular disease or chronic renal failure.
Perioperative Use:Administration of clonidine hydrochloride tablets should be continued to within four hours of surgery and resumed as soon as possible thereafter. Blood pressure should be carefully monitored during surgery and additional measures to control blood pressure should be available if required.
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
Toxicology).
Toxicology:In several studies with oral clonidine hydrochloride, a dose-dependent increase in the incidence and severity of spontaneous retinal degeneration was seen in albino rats treated for six months or longer. Tissue distribution studies in dogs and monkeys showed a concentration of clonidine in the choroid.
In view of the retinal degeneration seen in rats, eye examinations were performed during clinical trials in 908 patients before, and periodically after, the start of clonidine therapy. In 353 of these 908 patients, the eye examinations were carried out over periods of 24 months or longer. Except for some dryness of the eyes, no drug-related abnormal ophthalmological findings were recorded and, according to specialized tests such as electroretinography and macular dazzle, retinal function was unchanged.
In combination with amitriptyline, clonidine hydrochloride administration led to the development of corneal lesions in rats within 5 days.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Reproduction studies performed in rabbits at doses up to approximately 3 times the oral maximum recommended daily human dose (MRDHD) of clonidine hydrochloride tablets, USP produced no evidence of a teratogenic or embryotoxic potential in rabbits. In rats, however, doses as low as 1/3 the oral MRDHD (1/15 the MRDHD on a mg/m2 basis) of clodine were associated with increased resporptions in a study in which dams were trated continuously from 2 months prior to mating. Increased resporptions were not associated with treatment at the same time or at higher dose levels (up to 3 times the oral MRDHD) when the dams were treated on gestation days 6 to 15. Increases in resorption were observed at much higher dose levels (40 times the oral MRDHD on a mg/kg basis; 4 to 8 times the MRDHD on a mg.m2 basis) in mice and rats treated on gestation days 1 to 14 (lowest dose employed in the study was 500 mcg/kg).
No adequate, well-controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
NURSING MOTHERS
PEDIATRIC USE
WARNINGS,Withdrawal).CLONIDINE HYDROCHLORIDE ADVERSE REACTIONS
Body as a Whole:Fatigue, fever, headache, pallor, weakness, and withdrawal syndrome. Also reported were a weakly positive Coombstest and increased sensitivity to alcohol.
Cardiovascular:Bradycardia, congestive heart failure, electrocardiographic abnormalities (i.e., sinus node arrest, junctional bradycardia, high degree AV block and arrhythmias), orthostatic symptoms, palpitations, Raynaudphenomenon, syncope, and tachycardia. Cases of sinus bradycardia and atrioventricular block have been reported, both with and without the use of concomitant digitalis.
Central Nervous System:Agitation, anxiety, delirium, delusional perception, hallucinations (including visual and auditory), insomnia, mental depression, nervousness, other behavioral changes, paresthesia, restlessness, sleep disorder, and vivid dreams or nightmares.
Dermatological:Alopecia, angioneurotic edema, hives, pruritus, rash, and urticaria.
Gastrointestinal:Abdominal pain, anorexia, constipation, hepatitis, malaise, mild transient abnormalities in liver function tests, nausea, parotitis, pseudo-obstruction (including colonic pseudo-obstruction), salivary gland pain, and vomiting.
Genitourinary:Decreased sexual activity, difficulty in micturition, erectile dysfunction, loss of libido, nocturia, and urinary retention.
Hematologic:Thrombocytopenia.
Metabolic:Gynecomastia, transient elevation of blood glucose or serum creatine phosphokinase, and weight gain.
Musculoskeletal:Leg cramps and muscle or joint pain.
Oro-otolaryngeal:Dryness of the nasal mucosa.
Ophthalmological:Accommodation disorder, blurred vision, burning of the eyes, decreased lacrimation, and dryness of eyes.
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults:The dose of clonidine hydrochloride tablets, USP must be adjusted according to the patientindividual blood pressure response. The following is a general guide to its administration.Initial Dose:0.1 mg tablet twice daily (morning and bedtime). Elderly patients may benefit from a lower initial dose.
Maintenance Dose:Further increments of 0.1 mg per day may be made at weekly intervals if necessary until the desired response is achieved. Taking the larger portion of the oral daily dose at bedtime may minimize transient adjustment effects of dry mouth and drowsiness. The therapeutic doses most commonly employed have ranged from 0.2 mg to 0.6 mg per day given in divided doses.
Studies have indicated that 2.4 mg is the maximum effective daily dose, but doses as high as this have rarely been employed.
Renal Impairment:Dosage must be adjusted according to the degree of impairment, and patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
For questions regarding this product call Actavis at 1-800-432-8534.
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Clonidine HydrochlorideCLONIDINE HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!