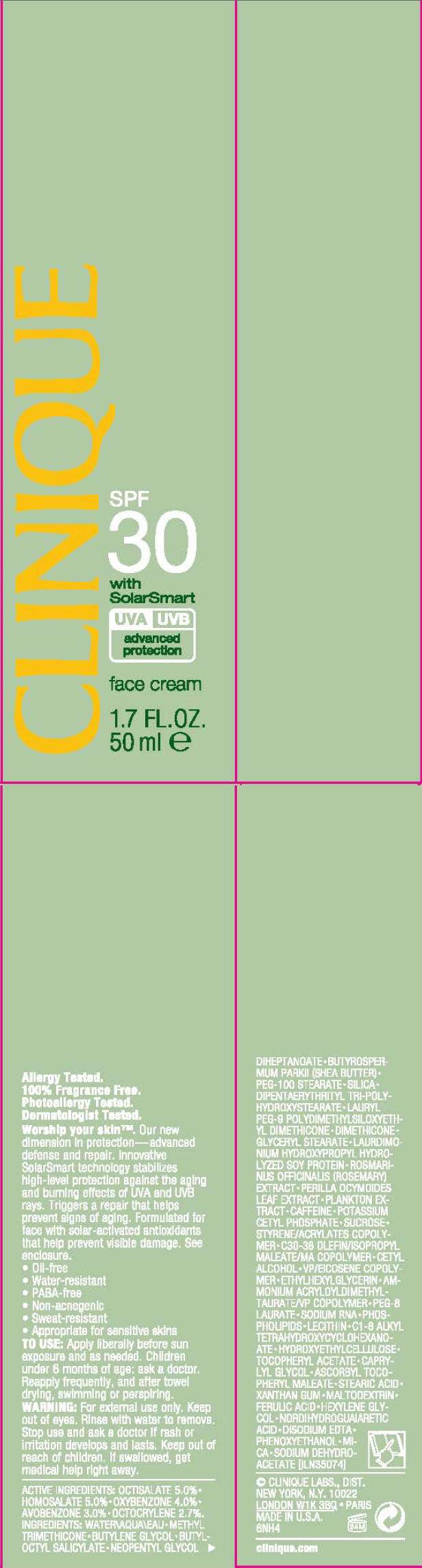
CLINIQUE
CLINIQUE SPF 30 with SolarSmart
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
TO USE
Apply liberally before sun exposure and as needed. Children under 6 months of age: ask a doctor. Reapply frequently, and after towel drying, swimming or perspiring.
WARNING
For external use only. Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help right away.
ACTIVE INGREDIENTS
OCTISALATE 5.0% • HOMOSALATE 5.0% • OXYBENZONE 4.0% • AVOBENZONE 3.0% • OCTOCRYLENE 2.7%.
INGREDIENTS
WATER • METHYL TRIMETHICONE • BUTYLENE GLYCOL • BUTYLOCTYL SALICYLATE • NEOPENTYL GLYCOL DIHEPTANOATE • BUTYROSPERMUM PARKII (SHEA BUTTER) • PEG-100 STEARATE • SILICA • DIPENTAERYTHRITYL TRI-POLYHYDROXYSTEARATE • LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE • DIMETHICONE • GLYCERYL STEARATE • LAURDIMONIUM HYDROXYPROPYL HYDROLYZED SOY PROTEIN • ROSMARINUS OFFICINALIS (ROSEMARY) EXTRACT • PERILLA OCYMOIDES LEAF EXTRACT • PLANKTON EXTRACT • CAFFEINE • POTASSIUM CETYL PHOSPHATE • SUCROSE • STYRENE/ACRYLATES COPOLYMER • C30-38 OLEFIN/ISOPROPYL MALEATE/MA COPOLYMER • CETYL ALCOHOL • VP/EICOSENE COPOLYMER • ETHYLHEXYLGLYCERIN • AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER • PEG-8 LAURATE • SODIUM RNA • PHOSPHOLIPIDS • LECITHIN • C1-8 ALKYL TETRAHYDROXYCYCLOHEXANOATE • HYDROXYETHYLCELLULOSE • TOCOPHERYL ACETATE • CAPRYLYL GLYCOL • ASCORBYL TOCOPHERYL MALEATE • STEARIC ACID • XANTHAN GUM • MALTODEXTRIN • FERULIC ACID • HEXYLENE GLYCOL • NORDIHYDROGUAIARETIC ACID • DISODIUM EDTA • PHENOXYETHANOL • MICA • SODIUM DEHYDROACETATE [ILN35074]
CLINIQUE LABS., DIST.
NEW YORK, N.Y. 10022
PRINCIPAL DISPLAY PANEL - 50 ml Carton
CLINIQUE
SPF
30
with
SolarSmart
UVA UVB
advanced
protection
face cream
1.7 FL. OZ.
50 ml e
CLINIQUEoctisalate, homosalate, oxybenzone, avobenzone, and octocrylene CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||