CLINIMIX E
CLINIMIX E sulfite-free(Amino Acid with Electrolytes in Dextrose with Calcium) Injections Ein CLARITY Dual Chamber Container
FULL PRESCRIBING INFORMATION: CONTENTS*
- CLINIMIX E DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINIMIX E INDICATIONS AND USAGE
- CLINIMIX E CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CLINIMIX E ADVERSE REACTIONS
- CLINIMIX E DOSAGE AND ADMINISTRATION
- DIRECTIONS FOR USE OF PLASTIC CONTAINER
- HOW SUPPLIED
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
CLINIMIX E DESCRIPTION
CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections are sterile, nonpyrogenic, hypertonic solutions in a CLARITY Dual Chamber Container.
The sulfite-free Amino Acid Injections with Electrolytes in the outlet port chamber are solutions of essential and nonessential amino acids provided with electrolytes.
The Dextrose Injections with Calcium in the injection port chamber are solutions for fluid replenishment and caloric supply.
After opening the seal between the chambers and mixing thoroughly, the admixed product is intended for intravenous use. See Table 1 for composition, pH, osmolarity, ionic concentration and caloric content of the admixed product.
The CLARITY Dual Chamber Container is a lipid-compatible plastic container (PL 2401 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
CLINICAL PHARMACOLOGY
CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections administered intravenously provide biologically utilizable source material for protein synthesis and have value as a source of calories, electrolytes, and water.
CLINIMIX E INDICATIONS AND USAGE
CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections are indicated as a caloric component in a parenteral nutrition regimen and as the protein (nitrogen) source for offsetting nitrogen loss or for treatment of negative nitrogen balance in patients where:
(1) the alimentary tract cannot or should not be used,
(2) gastrointestinal absorption of protein is impaired, or
(3) metabolic requirements for protein are substantially increased, as with extensive burns.
Central Vein Administration:
Central vein infusion should be used when amino acid solutions are admixed with hypertonic dextrose to promote protein synthesis such as for hypercatabolic or depleted patients or those requiring long term parenteral nutrition.
Peripheral Vein Administration:
For patients in whom the central vein route is not indicated, amino acid solutions diluted with low dextrose concentrations may be infused by peripheral vein.
CLINIMIX E CONTRAINDICATIONS
CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections are contraindicated in patients having intracranial or intraspinal hemorrhage, in patients who are severely dehydrated, in patients hypersensitive to one or more amino acids and in patients with severe liver disease or hepatic coma.
Solutions containing corn-derived dextrose may be contraindicated in patients with known allergy to corn or corn products.
WARNINGS
Additives may be incompatible including fat emulsions. Consult with pharmacist, if available.
When introducing additives, use aseptic techniques. Mix thoroughly.
Because of the potential for life-threatening events, caution should be taken to ensure that precipitates have not formed in any parenteral nutrient admixture.
These CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections, must be admixed prior to infusion. For admixing instructions see DIRECTIONS FOR USE OF PLASTIC CONTAINER.
The infusion of hypertonic nutrient injections into a peripheral vein may result in vein irritation, vein damage, and thrombosis. After mixing, strongly hypertonic nutrient injections should only be administered through an indwelling intravenous catheter with the tip located in a large central vein, such as the superior vena cava.
Proper administration of these admixed amino acid with electrolytes/dextrose with calcium injections requires a knowledge of fluid and electrolyte balance and nutrition as well as clinical expertise in recognition and treatment of the complications which may occur.
Laboratory Tests
Frequent clinical evaluation and laboratory determinations are necessary for proper monitoring during administration. Studies should include blood sugar, serum proteins, kidney and liver function tests, electrolytes, complete blood count with differential, carbon dioxide combining power or content, serum osmolarities, blood cultures, and blood ammonia levels.
Administration of amino acid solutions to a patient with hepatic insufficiency may result in serum amino acid imbalances, hyperammonemia, stupor, and coma.
Hyperammonemia is of special significance in infants. This reaction appears to be related to a deficiency of the urea cycle amino acids of genetic or product origin. It is essential that blood ammonia be measured frequently in infants.
Conservative doses of these admixed amino acid with electrolytes/dextrose with calcium injections should be given to patients with known or suspected hepatic dysfunction. Should symptoms of hyperammonemia develop, administration should be discontinued and the patient’s clinical status be reevaluated.
Administration of amino acid solutions in the presence of impaired renal function presents special issues associated with retention of electrolytes.
These admixed injections should not be administered simultaneously with blood through the same infusion set because of the possibility of pseudoagglutination.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
PRECAUTIONS
With the administration of these CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections, hyperglycemia, glycosuria, and hyperosmolar syndrome may result. Blood and urine glucose should be monitored on a routine basis in patients receiving this therapy.
Use with caution when administering to patients with anuria or renal failure.
These injections contain sufficient electrolytes to provide for most parenteral nutritional needs with the possible exception of potassium, where supplementation may be required. However, replacement of exceptional electrolyte loss due to nasogastric suction, fistula drainage, or unusual tissue exudation may be necessary. Particular attention should be given to monitoring serum potassium levels.
The metabolizable acetate anion and amino acid profiles in these admixed injections were designed to minimize or prevent occurrences of hyperchloremic metabolic acidosis and hyperammonemia. However, the physician should be aware of appropriate countermeasures if they become necessary.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Because of its anti-anabolic activity, concurrent administration of tetracycline may reduce the protein-sparing effect of infused amino acids.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states, or pulmonary edema; particularly in patients with renal disease, pulmonary insufficiency, and heart disease.
Administration of admixed amino acid with electrolytes/dextrose with calcium injections and other nutrients via central or peripheral venous catheter may be associated with complications which can be prevented or minimized by careful attention to all aspects of the procedure. This includes attention to solution preparation, administration, and patient monitoring. It is essential that a carefully prepared protocol based on current medical practices be followed, preferably by an experienced team.
Although a detailed discussion of the complications is beyond the scope of this insert, the following summary lists those based on current literature:
Technical:
The placement of a central venous catheter should be regarded as a surgical procedure. The physician should be fully acquainted with various techniques of catheter insertion as well as recognition and treatment of complications. For details of techniques and placement sites, consult the medical literature. X-ray is the best means of verifying catheter placement. Complications known to occur from the placement of central venous catheters are pneumothorax, hemothorax, hydrothorax, artery puncture and transection, injury to the brachial plexus, malposition of the catheter, formation of arteriovenous fistula, phlebitis, thrombosis, cardiac arrhythmia, and catheter embolus.
Septic:
The constant risk of sepsis is present during total parenteral nutrition. Since contaminated solutions and infusion catheters are potential sources of infection, it is imperative that the preparation of solution and the placement and care of catheters be accomplished under controlled aseptic conditions. If fever develops, the solution, its delivery system, and the site of the indwelling catheter should be changed.
Metabolic:
The following metabolic complications have been reported: metabolic acidosis, hypophosphatemia, alkalosis, hyperglycemia and glycosuria, osmotic diuresis and dehydration, rebound hypoglycemia, elevated liver enzymes, hypo- and hypervitaminosis, electrolyte imbalances, and hyperammonemia. Frequent clinical evaluation and laboratory determinations are necessary, especially during the first few days of therapy to prevent or minimize these complications.
Caution must be exercised in the administration of these admixed amino acid with electrolytes/dextrose with calcium injections to patients receiving corticosteroids or corticotropin.
These admixed injections should be used with caution in patients with overt or known subclinical diabetes mellitus.
Drug product contains no more than 25 mcg/L of aluminum.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy:
Teratogenic Effects
Pregnancy Category C.
Animal reproduction studies have not been conducted with CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections. It is also not known whether CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
Caution should be exercised when CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections are administered to a nursing woman.
Pediatric Use:
Dextrose is safe and effective for the stated indications in pediatric patients (see INDICATIONS AND USAGE). As reported in the literature, the dosage selection and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia. Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
Safety and effectiveness of CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections in pediatric patients have not been established by adequate and well-controlled studies. However, the use of amino acid injections in pediatric patients as an adjunct in the offsetting of nitrogen loss or in the treatment of negative nitrogen balance is referenced in the medical literature. See DOSAGE AND ADMINISTRATION.
Geriatric Use:
Clinical studies of CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from other younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or drug therapy.
CLINIMIX E ADVERSE REACTIONS
See WARNINGS and PRECAUTIONS
Too rapid infusion of these CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections may result in diuresis, hyperglycemia, glycosuria, and hyperosmolar coma. Continual clinical monitoring of the patient is necessary in order to identify and initiate measures for these clinical conditions.
Reactions that may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation, and hypervolemia. Policies and procedures should be established for the recognition and management of such reactions.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
CLINIMIX E DOSAGE AND ADMINISTRATION
If a patient is unable to take oral nourishment for a prolonged period of time, institution of total parenteral nutrition should be considered.
The total daily dose of CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections depends on the patient’s metabolic requirement and clinical response. The determination of nitrogen balance and accurate daily body weights, corrected for fluid balance, are probably the best means of assessing individual nitrogen requirements.
Recommended Dietary Allowances Food and Nutrition Board National Academy of Sciences - National Research Council (Revised 1989).
For the initial treatment of trauma or protein calorie malnutrition, higher doses of protein with corresponding quantities of carbohydrates will be necessary to promote adequate patient response to therapy. The severity of the illness being treated is the primary consideration in determining proper dose level. Such higher doses, especially in infants, must be accompanied by more frequent laboratory evaluation.
Care should be exercised to insure the maintenance of proper levels of serum potassium. Quantities of 60 to 180 mEq of potassium per day have been used with adequate clinical effect. It may be necessary to add quantities of this electrolyte to these admixed injections, depending primarily on the amount of carbohydrate administered to and metabolized by the patient.
Total daily fluid requirements can be met beyond the volume of amino acids solution by supplementing with noncarbohydrate or carbohydrate-containing electrolyte solutions.
Maintenance vitamins, additional electrolytes, and trace elements should be administered as required.
In many patients, provision of adequate calories in the form of hypertonic dextrose may require the administration of exogenous insulin to prevent hyperglycemia and glycosuria.
Fat emulsion administration should be considered when prolonged (more than 5 days) parenteral nutrition is required in order to prevent essential fatty acid deficiency (EFAD). Serum lipids should be monitored for evidence of EFAD in patients maintained on fat-free TPN.
Intravenous fat emulsions provide approximately 1.1 kcal per mL (10%), 2.0 kcal per mL (20%), or 3.0 kcal per mL (30%) and may be admixed along with amino acid with electrolytes/dextrose with calcium injections in the CLARITY Container to supplement caloric intake.
Depending upon the clinical condition of the patient, approximately 3 liters of solution may be administered per 24 hour period. When used postoperatively, the therapy should begin with 1000 mL on the first postoperative day. Thereafter, the dose may be increased to 3000 mL per day.
Do not administer unless seal between chambers is opened, other seals are intact, and solution is clear and thoroughly mixed.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions, where possible.
A slight yellow color does not alter the quality and efficacy of this product.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available. If, in the informed judgment of the physician, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced.
Do not store solutions containing additives. These amino acid with electrolytes/dextrose with calcium injections should be used promptly after mixing. Any storage with additives should be under refrigeration and limited to a brief period of time, less than 24 hours.
Pediatric Use:
Use of CLINIMIX E sulfite-free (Amino Acid with Electrolytes in Dextrose with Calcium) Injections in pediatric patients is governed by the same considerations that affect the use of any amino acid solution in pediatrics. The amount administered is dosed on the basis of grams of amino acids/kg of body weight/day. Two to 3 g/kg of body weight for infants with adequate calories are generally sufficient to satisfy protein needs and promote positive nitrogen balance. Solution administrations by peripheral vein should not exceed twice normal serum osmolarity (718 mOsmol/L).
Central Vein Administration:
Hypertonic mixtures of amino acid with electrolytes/dextrose with calcium injections may be administered safely by continuous infusion through a central vein catheter with the tip located in the vena cava. In addition to meeting nitrogen needs, the administration rate is governed, especially during the first few days of therapy, by the patient’s tolerance to dextrose, as indicated by frequent determinations of urine and blood sugar levels. Daily intake of amino acid with electrolytes/dextrose with calcium injections should be increased gradually to the maximum required dose.
Sudden cessation in administration of these admixed injections may result in insulin reaction due to continued endogenous insulin production. Parenteral nutrition mixtures should be withdrawn slowly.
Peripheral Vein Administration:
For patients requiring parenteral nutrition in whom the central vein route is not indicated, low concentration amino acid with electrolytes/dextrose with calcium injections may be administered by peripheral vein. In pediatric patients, the final solution should not exceed twice normal serum osmolarity (718 mOsmol/L).
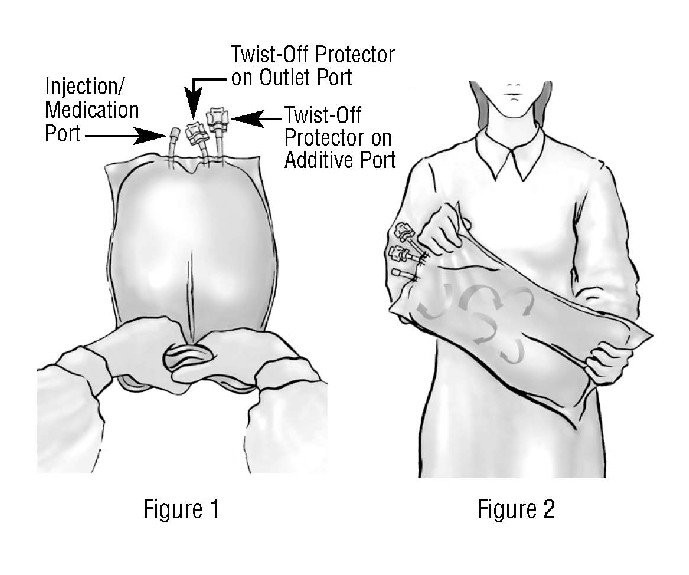
DIRECTIONS FOR USE OF PLASTIC CONTAINER
WARNING: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
BE SURE THE CONTENTS OF BOTH CHAMBERS ARE MIXED TOGETHER AFTER OPENING SEAL BETWEEN CHAMBERS. After opening seal between chambers, lipids and/or additives can be introduced to the container. Thorough mixing ensures complete delivery of all ingredients.
To Open
Tear overwrap across top at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
Check to ensure seal between chambers is intact, i.e., solutions are contained in separate chambers. Check for minute leaks by separately squeezing each chamber. If external leaks or leakage between the chambers are found, discard solution as sterility or stability may be impaired.
To Mix Solutions
Grasp the container firmly on each side of the top of the bag and roll bag to open seal between chambers as shown in Figure 1. Mix solutions thoroughly as shown in Figure 2. Check for leaks.
Storage
If removed from the overwrap and the contents are not mixed, CLINIMIX E Injection solutions may be stored under refrigeration for up to 9 days.
Upon mixing of bag contents, CLINIMIX E Injection solutions remain stable when stored under refrigeration, not to exceed 9 days from when the product was originally removed from the overwrap.
CLINIMIX E Injection solutions containing additives should be used promptly after admixture. Any storage should be under refrigeration and limited to a brief period of time, less than 24 hours.
To add Fat Emulsion for 3-in-1 admixture:
See WARNINGS section regarding incompatible additives including fat emulsions.
- Prior to adding fat emulsion, mix amino acid and dextrose injection as shown in Figure 2.
- Prepare fat emulsion transfer set following instructions provided.
- Attach transfer set to fat emulsion bottle using aseptic technique.
- Twist off protector on the additive port of the CLARITY container.
- Attach the transfer set to the exposed additive port.
- Open clamp on transfer set.
- After completing transfer, use appropriate plastic clamp or metal ferrule to seal off additive port tube.
- Remove transfer set.
- Mix contents of CLARITY container thoroughly. Check for leaks.
Storage: Storage of the 3-in-1 admixture must be under refrigeration and limited to a brief period of time, no longer than 24 hours. See WARNINGS section regarding incompatible additives.
To Add Medication
WARNING: Additives may be incompatible.
Supplemental medication may be added with a 19 to 22 gauge needle through the medication port.
- Prepare medication port.
- Using syringe with 19 to 22 gauge needle, puncture resealable medication port and inject.
- Mix solution and medication thoroughly. For high density medication, such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
- Check for leaks.
Preparation for Administration
- Suspend container from eyelet support.
- Twist off protector from outlet port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
HOW SUPPLIED
See Table 1.
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C/77°F): brief exposure up to 40°C/104°F does not adversely affect the product.
Refrigerated storage is limited to 9 days once overwrap has been opened.
Do not use if overwrap has been previously opened or damaged.
| Composition | ||||||||||||||||||||
| Essential Amino Acids (mg/100mL) | Nonessential Amino Acids (mg/100mL) | |||||||||||||||||||
| How Supplied | ||||||||||||||||||||
| After mixing, the product represents |
1000 mL Code and NDC Number |
2000 mL Code and NDC Number |
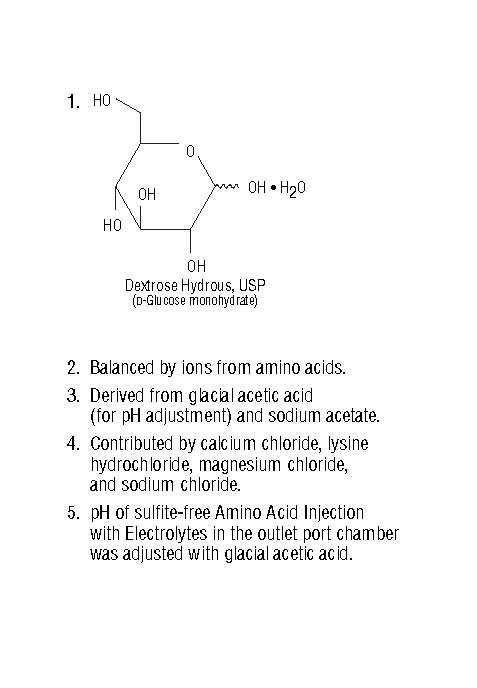
Dextrose Hydrous, USP1(g/100mL) | Amino Acids (g/100mL) | Total Nitrogen (mg/100mL) | Leucine - (CH3)2 CHCH2CH (NH2) COOH | Isoleucine - CH3CH2CH (CH3) CH (NH2) COOH | Valine - (CH3)2 CHCH (NH2) COOH |
Lysine (added as the hydrochloride salt) - H2N (CH2)4 CH (NH2) COOH |
Phenylalanine - (C6H5) CH2 CH (NH2) COOH | Histidine - (C3H3N2) CH2CH (NH2) COOH | Threonine - CH3CH (OH) CH (NH2) COOH |
Methionine - CH3S (CH2)2 CH (NH2) COOH |
Tryptophan - (C8H6N) CH2 CH (NH2) COOH | Alanine - CH3CH (NH2) COOH | Arginine - H2NC (NH) NH (CH2)3 CH (NH2) COOH | Glycine - H2NCH2COOH | Proline - [(CH2)3 NH CH] COOH | Serine - HOCH2CH (NH2) COOH | Tyrosine - [C6H4 (OH)] CH2CH (NH2) COOH |
| CLINIMIX E 2.75/5 sulfite-free (2.75% Amino Acid with Electrolytes in 5% Dextrose with Calcium) Injection | Code 2B7735 NDC 0338-1142-03 |
Code 2B7713 NDC 0338-1107-04 |
5 | 2.75 | 454 | 201 | 165 | 160 | 159 | 154 | 132 | 116 | 110 | 50 | 570 | 316 | 283 | 187 | 138 | 11 |
| CLINIMIX E 2.75/10 sulfite-free (2.75% Amino Acid with Electrolytes in 10% Dextrose with Calcium) Injection | Code 2B7736 NDC 0338-1143-03 |
Code 2B7714 NDC 0338-1109-04 |
10 | 2.75 | 454 | 201 | 165 | 160 | 159 | 154 | 132 | 116 | 110 | 50 | 570 | 316 | 283 | 187 | 138 | 11 |
| CLINIMIX E 4.25/5 sulfite-free (4.25% Amino Acid with Electrolytes in 5% Dextrose with Calcium) Injection | Code 2B7737 NDC 0338-1144-03 |
Code 2B7716 NDC 0338-1113-04 |
5 | 4.25 | 702 | 311 | 255 | 247 | 247 | 238 | 204 | 179 | 170 | 77 | 880 | 489 | 438 | 289 | 213 | 17 |
| CLINIMIX E 4.25/10 sulfite-free (4.25% Amino Acid with Electrolytes in 10% Dextrose with Calcium) Injection | Code 2B7738 NDC 0338-1145-03 |
Code 2B7717 NDC 0338-1115-04 |
10 | 4.25 | 702 | 311 | 255 | 247 | 247 | 238 | 204 | 179 | 170 | 77 | 880 | 489 | 438 | 289 | 213 | 17 |
| CLINIMIX E 4.25/25 sulfite-free (4.25% Amino Acid with Electrolytes in 25% Dextrose with Calcium) Injection | Code 2B7739 NDC 0338-1146-03 |
Code 2B7719 NDC 0338-1119-04 |
25 | 4.25 | 702 | 311 | 255 | 247 | 247 | 238 | 204 | 179 | 170 | 77 | 880 | 489 | 438 | 289 | 213 | 17 |
| CLINIMIX E 5/15 sulfite-free (5% Amino Acid with Electrolytes in 15% Dextrose with Calcium) Injection | Code 2B7740 NDC 0338-1147-03 |
Code 2B7721 NDC 0338-1123-04 |
15 | 5 | 826 | 365 | 300 | 290 | 290 | 280 | 240 | 210 | 200 | 90 | 1035 | 575 | 515 | 340 | 250 | 20 |
| CLINIMIX E 5/20 sulfite-free (5% Amino Acid with Electrolytes in 20% Dextrose with Calcium) Injection | Code 2B7741 NDC 0338-1148-03 |
Code 2B7722 NDC 0338-1125-04 |
20 | 5 | 826 | 365 | 300 | 290 | 290 | 280 | 240 | 210 | 200 | 90 | 1035 | 575 | 515 | 340 | 250 | 20 |
| CLINIMIX E 5/25 sulfite-free (5% Amino Acid with Electrolytes in 25% Dextrose with Calcium) Injection | Code 2B7742 NDC 0338-1149-03 |
Code 2B7723 NDC 0338-1127-04 |
25 | 5 | 826 | 365 | 300 | 290 | 290 | 280 | 240 | 210 | 200 | 90 | 1035 | 575 | 515 | 340 | 250 | 20 |
| Composition | ||||||||||||||||||||
| Electrolytes (mg/100mL) | Electrolyte Profile (mEq/L)2 | Caloric Content (kcal/L) | ||||||||||||||||||
| Sodium Acetate Trihydrate, USP - C2H3NaO2•3H2O | Dibasic Potassium Phosphate, USP - K2HPO4 | Sodium Chloride, USP - NaCl | Magnesium Chloride, USP - MgCl2•6H2O | Calcium Chloride Dihydrate, USP - CaCl2•2H2O | Sodium | Potassium | Magnesium | Calcium | Acetate3 | Chloride4 |
Phosphate (as HPO4 -) |
pH5
(range) |
Osmolarity (mOsmol/L)(calc) | From Dextrose | From Amino Acids | TOTAL (Dextrose and Amino Acids) | ||||
| 217 | 261 | 112 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
51 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
665 | 170 | 110 | 280 | ||||
| 217 | 261 | 112 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
51 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
920 | 340 | 110 | 450 | ||||
| 297 | 261 | 77 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
70 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
815 | 170 | 170 | 340 | ||||
| 297 | 261 | 77 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
70 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
1070 | 340 | 170 | 510 | ||||
| 297 | 261 | 77 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
70 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
1825 | 850 | 170 | 1020 | ||||
| 340 | 261 | 59 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
80 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
1395 | 510 | 200 | 710 | ||||
| 340 | 261 | 59 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
80 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
1650 | 680 | 200 | 880 | ||||
| 340 | 261 | 59 | 51 | 33 | 35 | 30 | 5 | 4.5 (2.2 mmol/L) |
80 | 39 | 30 (15 mmol/L) |
6.0 (4.5 to 7.0) |
1900 | 850 | 200 | 1050 | ||||
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
Baxter, Clinimix E, and Clarity are trademarks of Baxter International Inc.
07-19-57-385
Rev. July 2010
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL

Lot: xxxxx
Exp: xxx xx
QTY: 6 PK 500mL/500mL
Code: 2B7738
NDC 0338-1145-03
CLINIMIX E 4.25/10
(4.25% Amino Acid w/ ELECTROLYTES In 10% Dextrose w/ Calcium) INJ
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CLINIMIX ELeucine, Phenylalanine, Lysine, Methionine, Isoleucine, Valine, Histidine, Threonine, Tryptophan, Alanine, Glycine, Arginine, Proline, Serine, Tyrosine, Sodium Acetate, Dibasic Potassium Phosphate, Magnesium Chloride, Sodium Chloride, Calcium Chloride, Dextrose INJECTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||